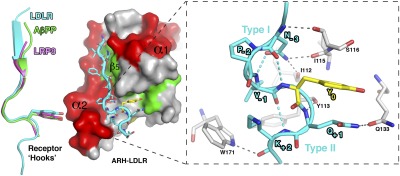
Fig. 2.
LDLR adopts a unique “Hook”-like structure. Left: Structure of the ARH–LDLR peptide complex. The LDLR receptor tail adopts a “Hook” shape formed by an N-terminal β-strand followed by a type I β-turn. This turn is characterized by an intra H-bond between the asparagine (N-3) and the tyrosine (Y0) as seen for other, albeit shorter, NPxY receptor tails overlaid on the LDLR tail on the left (LRP8 in magenta and AβPP in green; PDB codes 1NU2 and 1M7E, respectively). However, a close-up view (Right) shows that the type I β-turn of LDLR is followed by a consecutive type II β-turn, and each turn is supported by multiple intra- (cyan) and inter- (gray) hydrogen bonds, indicated as dashed lines.