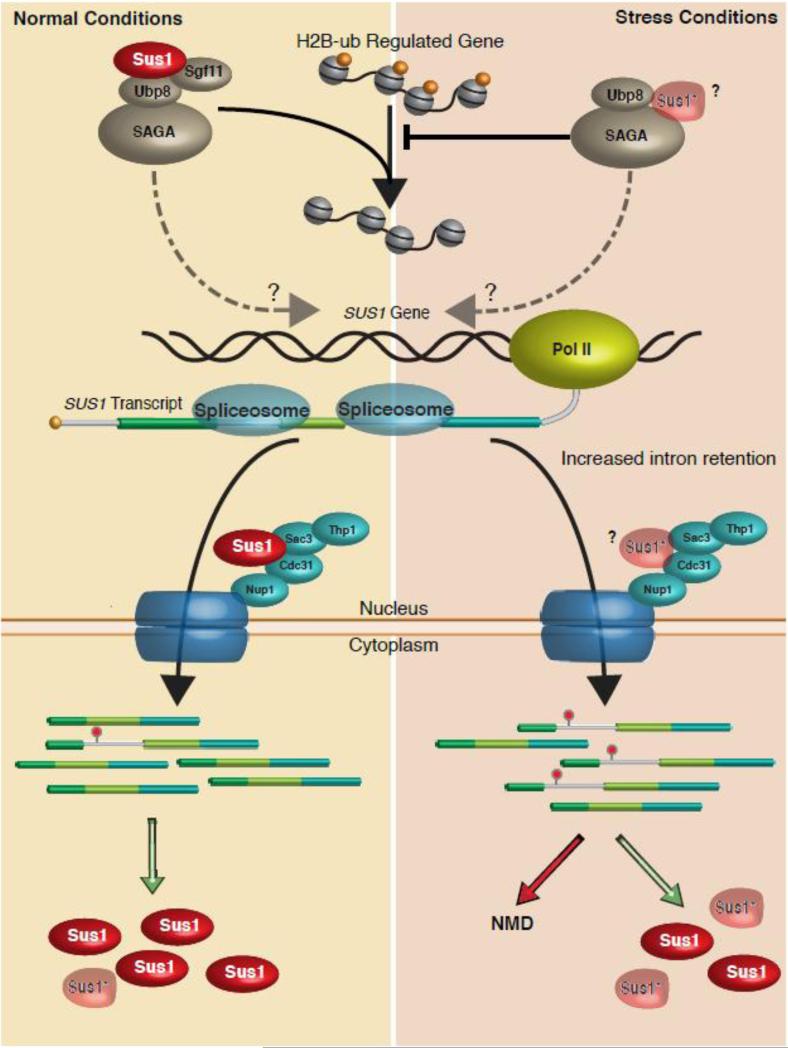
Figure 3.
Regulated splicing of SUS1 fine-tunes RNA export and histone H2B ubiquitination. Under unstressed conditions (Left side of figure) SUS1 pre-mRNA undergoes splicing and is exported to allow production of Sus1 protein that can either participate in histone H2B deubiquitination as part of the SAGA complex (top) or can associate with the TREX-2 proteins at the nuclear pore (Shown in dark blue). Intron 1 is retained in a small fraction of the RNAs and is either targeted to NMD or may make a truncated product. Under stress conditions, intron 1retention is enhanced. However, it is unclear how much of this product is targeted to NMD vs. translation of a truncated product. It is possible that the truncated protein can associate with either the TREX-2 complex or with SAGA (indicated by “?”). Sgf11 is destabilized by the lack of Sus1 [81], so it has been left off out of the SAGA complex that contains less SUS1. It is also possible that Sus1, via its H2B ubiquitination activity or by interacting with the splicing (not shown), may influence its own splicing (Dotted arrow).