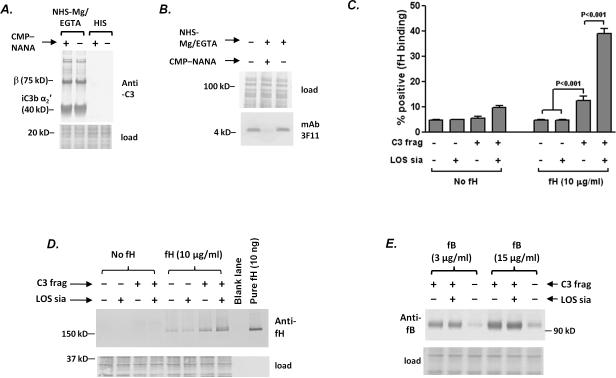
Figure 3.
LOS sialylation enhances human fH binding to C3 fragments deposited on meningococci, but does not affect factor B interactions with surface-bound C3b. A. C3 deposition (detected with goat polyclonal anti-human C3) on A2594 ΔfHbpΔNspA bacteria that were incubated with NHS-Mg/EGTA (20% (v/v) and subsequently grown in the presence or absence of CMP-NANA. Bacteria incubated with heat inactivated serum (HIS) were used as controls. Proteins below the 40 kD marker were stained with Coomassie blue (“load”) and served as a loading control. B. LOS sialylation following growth of bacteria in NHS-Mg/EGTA. Sialylation of the LOS A2594 ΔfHbpΔNspA incubated with NHS-Mg/EGTA and then grown in the presence of CMP-NANA was confirmed by western blotting with mAb 3F11 that recognizes the unsialylated lacto-N-neotetraose LOS; mAb 3F11 does not recognize sialylated LOS. Proteins above the 50 kD marker were stained with Coomassie blue served as a loading control. C. LOS sialylation augments binding of full length fH to meningococci coated with C3 fragments. Aliquots of bacteria that were incubated with NHS-Mg/EGTA or HIS (controls) were grown either in the presence or absence of CMP-NANA, followed by incubation with purified human fH (10 μg/ml) or buffer alone (no fH). fH bound to bacteria was detected by flow cytometry using affinity-isolated polyclonal goat anti-human fH. The percentage of positive events in each sample was measured relative to control reaction (bacteria without C3, LOS sialic acid or added fH). Each bar represents the mean (SEM) of 5 independent observations. Comparisons across groups was made using 1-way ANOVA. D. Western blotting to demonstrate that LOS sialylation augments binding of full length factor H to meningococci coated with C3 fragments. Bacteria (A2594 ΔfHbpΔNspA) were coated with C3 fragments and incubated with human fH, as in C. Controls included bacteria that lacked C3 and/or added human fH. Bacteria were washed, lysed and proteins separated on a 4–12% Bis-Tris gel followed by western blotting. Proteins below the 50 kD marker were stained with Coomassie blue to measure bacterial lysate loading. This experiment was repeated thrice separately with similar results. E. LOS sialylation does not affect factor B binding to C3 fragments deposited on meningococci. Strain A2594 ΔfHbpΔNspA was incubated with NHS-Mg/EGTA (20%) that contained an anti-factor I mAb to decrease conversion of C3b to iC3b and then grown in media that either contained or lacked CMP-NANA as in C. Organisms incubated with heat-inactivated serum (HIS) served as a control. Bacteria were then incubated with purified factor B (fB) at concentrations of 3 μg/ml or 15 μg/ml and factor B bound to bacteria was measured by western blotting..