Figure 5.
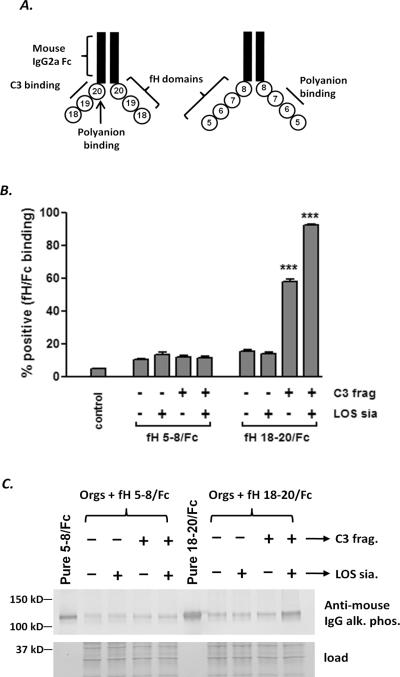
Meningococcal LOS sialylation selectively enhances the interaction of the C-terminal domains of human fH with C3 fragments on the bacterial surface. A. Schematic of two fH/Fc fusion proteins used in this study. Contiguous domains of human fH were fused to murine IgG2a Fc. The locations of the C3d binding regions (domains 19–20) and polyanion binding sites (domains 6, 7 and 20) in each of the molecules are indicated. B. LOS sialylation selectively enhances binding of fH 18–20/Fc to C3b/iC3b-coated meningococci. Bacteria (A2594 ΔfHbpΔNspA) were incubated with NHS-Mg/EGTA (20% (v/v)), sialylated as described in Fig. 3 and then incubated with either fH 5–8/Fc or fH 18–20/Fc (10 μg/ml of each protein). Bacteria incubated with HIS were used as controls. Bound fH/Fc was detected with anti-mouse IgG conjugated to FITC. The percentage of positive events was determined relatie to bacteria incubated with HIS alone. Each bar represents the mean (SEM) of 5 separate observations and data sets were compared by 1-way ANOVA. C. Western blotting to show that LOS sialic acid and C3 fragments together cooperate to enhance the interaction of the C-terminus of human fH with meningococci. Samples were prepared as in B, washed and western blotted and bound fH/Fc was detected with anti-mouse IgG conjugated to alkaline phosphatase. This experiment was performed twice with similar results.