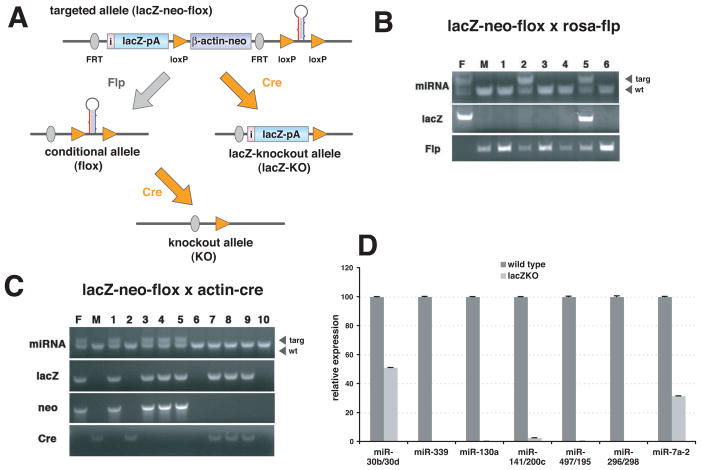
Figure 2. Generation of knockout mice.
(A) Breeding strategy. Combination of FRT and loxP sites allows manipulation of targeted allele in mice. Germline-transmitted mice (lacZ-neo-flox) can be crossed with germline deleter Flp mice, which will remove the reporter and the neomycin cassette to restore the wild type allele (conditional allele/flox). Then conditional mice can be crossed with either germline- or tissue-specific Cre transgenic mice to generate knockouts (knockout allele/KO). Instead of sequential recombination steps, germline-transmitted mice can be crossed with germline deleter Cre mice to produce mice with a reporter-tagged null allele (lacZ-knockout allele/lacZ-KO). (B) Flp-mediated recombination of FRT sites in mice. Germline-transmitted mice were crossed with homozygous Rosa-Flp mice. Parents and offspring were PCR-genotyped to check deletion of the lacZ reporter. Flp excision was not fully penetrant as the lacZ was not deleted in #5 pup, although it was completely excised in #2 pup (F and M indicate father and mother respectively). (C) Cre-mediated recombination of loxP sites in mice. Germline-transmitted mice were crossed with actin-Cre transgenic mice. Parents and offspring were PCR-genotyped to evaluate deletion of the neomycin marker, but not of the lacZ reporter. Excision by actin-Cre was complete among all pups (#7 to #9). (D) qPCR analysis to confirm the loss of miRNA in lacZ-KO mice. Either lung (miR-30b/30d, miR-339, miR-130a, miR-141/200c, miR-479/195 and miR-296/298) or brain (miR-7a-2) of 2 to 6 month-old knockout mice was used to isolate total RNA. RT-PCR was done by using Taqman miRNA assays. PCR were done as triplicates and data were presented as means with standard deviation.