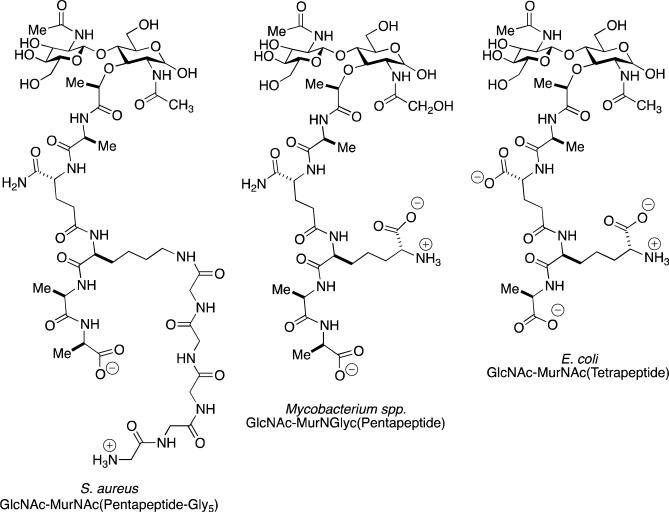
Scheme 1.
Left: A representative muropeptide of the monoderm Gram-positive bacterium Staphylococcus aureus. The GlcNAc-MurNAc disaccharide is shown as the “free” MurNAc glycoside. This reducing saccharide is customarily transformed by chemical reduction prior to chromatographic analysis. The sequence of the peptide stem attached to the lactyl carboxylate of the MurNAc is l-Ala-d-iGln-l-Lys-d-Ala-d-Ala. A (Gly)5 “bridge” is biosynthetically added to the ε-amine of the l-Lys. In the stem cross-linking event catalyzed by the bifunctional penicillin-binding protein enzymes, the ultimate d-Ala residue is lost as a leaving group as an acyl-enzyme is formed. This d-alanyl acyl-enzyme is transferred to the amine of the terminal glycine of the (Gly)5 bridge of an adjacent peptidoglycan strand to complete the cross-link. Center: A representative muropeptide of a Gram-positive Mycobacterium spp., with an l-Ala-d-iGln-meso-DAP-d-Ala-d-Ala stem peptide sequence. Crosslinking occurs from the d-alanyl acyl-enzyme derived from one strand to the amine terminus of the meso-diaminopimelate of an adjacent strand. Right: A representative muropeptide of the diderm Gram-negative bacterium Escherichia coli.