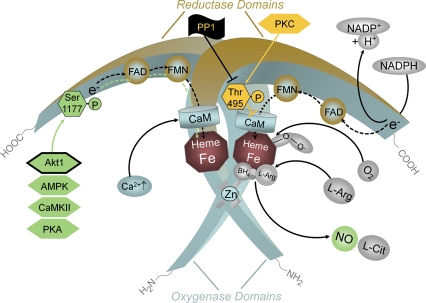
Figure 3.
Regulation of endothelial NOS activity by intracellular Ca2+ and phosphorylation. An increase in intracellular Ca2+ leads to an enhanced binding of calmodulin (CaM) to the enzyme, which in turn displaces an auto-inhibitory loop and facilitates the flow of electrons from NADPH in the reductase domain to the haem in the oxygenase domain. Established functionally important phosphorylation sites in human endothelial NOS are Ser1177 and Thr495. In resting endothelial cells, Ser1177 is usually not phosphorylated. Phosphorylation is induced when the cells are exposed to oestrogens, vascular endothelial growth factor (VEGF), insulin, bradykinin or fluid shear stress. The kinases responsible for phosphorylation (green hexagons) depend on the primary stimulus. Oestrogen and vascular endothelial growth factor elicit phosphorylation of Ser1177 by activating serine/threonine kinase Akt. So far, Akt1 is the only kinase proven to regulate endothelial NOS function in vivo (framed green hexagon). Insulin probably activates both Akt and the AMP-activated protein kinase (AMPK), the bradykinin-induced phosphorylation of Ser1177 is mediated by Ca2+/calmodulin-dependent protein kinase II (CaMKII), and shear stress leads to phosphorylation of endothelial NOS mainly via protein kinase A (PKA). Phosphorylation of the Ser1177 residue increases the flux of electrons through the reductase domain and thus enzyme activity. The Thr495 residue of human endothelial NOS tends to be constitutively phosphorylated in endothelial cells. Thr495 is a negative regulatory site, and its phosphorylation is associated with a decreased electron flux and enzyme activity. The constitutively active kinase that phosphorylates endothelial NOS Thr495 is most probably protein kinase C (PKC, yellow hexagon). Phosphorylation of Thr495 reduces endothelial NOS activity (yellow block arrow). The phosphatase that dephosphorylates Thr495 appears to be protein phosphatase1 (PP1, black flag with black block arrow).