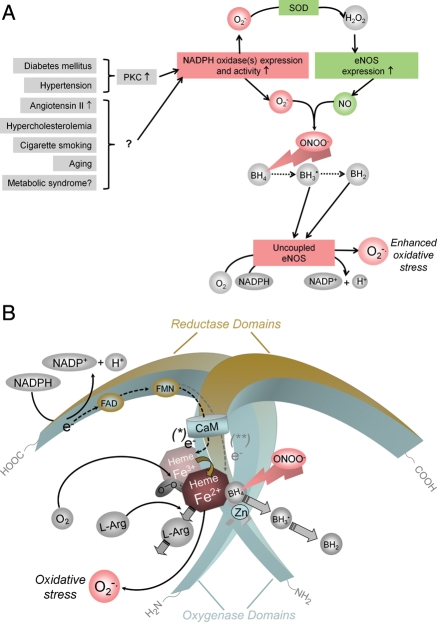
Figure 4.
Potential mechanisms by which cardiovascular risk factors lead to oxidative stress and endothelial NOS uncoupling. (A) In many types of vascular disease, NADPH oxidases are up-regulated in the vascular wall and generate superoxide (O2−•). In experimental diabetes mellitus and angiotensin II-induced hypertension, this has been shown to be mediated by protein kinase C (PKC).168,169 Expression of endothelial NOS is also increased in vascular disease. H2O2, the dismutation product of O2−• can increase endothelial NOS expression via transcriptional and post-transcriptional mechanisms (SOD, superoxide dismutase).170 In addition, also protein kinase C activation can enhance endothelial NOS expression,171 and protein kinase C inhibitors reduce endothelial NOS expression levels in vascular disease.169 The products of NADPH oxidases and endothelial NOS, O2−• and NO·, rapidly recombine to form peroxynitrite (ONOO−). This can oxidize the essential cofactor of endothelial NOS (6R-)5,6,7,8-tetrahydrobiopterin (BH4) to trihydrobiopterin radical (BH3•).172,173 BH3• can disproportionate to the quinonoid 6,7-[8H]-H2-biopterin (BH2). As a consequence, oxygen reduction and O2 reduction by endothelial NOS are uncoupled from NO· formation, and a functional NOS is converted into a dysfunctional O2−•-generating enzyme that contributes to vascular oxidative stress. The enhanced endothelial NOS expression (see above) aggravates the situation. (B) Oxidation of BH4 to biologically inactive products such as the BH3• radical or BH2 also reduces the affinity of the substrate l-arginine (l-Arg) to NO, and NOS catalyzes the uncoupled reduction in O2, leading to the production of O2−• (and possibly also H2O2).