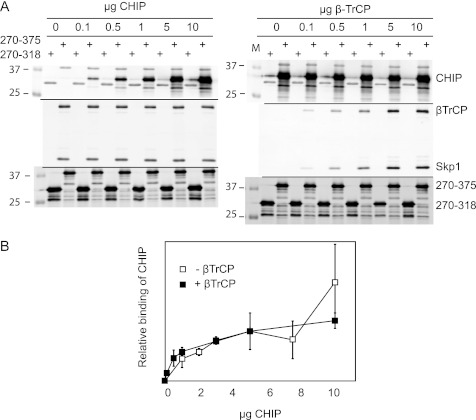
FIGURE 5.
CHIP binds GHR independently of βTrCP. A, glutathione beads were maximally loaded with recombinant GST-GHR270–375 or GST-GHR270–318 (illustrated in the lower panels). The beads were washed and incubated with saturating concentrations (10 μg/ml) of either purified βTrCP (left panels) or CHIP (right panels). The beads were extensively washed and incubated with indicated concentrations of competing ligand (CHIP, left panels; βTrCP, right panels) for 2 h at 4 °C. In both cases, maximal binding was achieved at 5–10 μg/ml similar to conditions without competing ligands. The antibodies used for detection are indicated in the figure. Binding was monitored by Western blotting. GST-GHR270–318 served as background binding. B, the effect of βTrCP on the binding of CHIP to GST-GHR270–375 was quantified. Glutathione beads saturated with GST-GHR270–375 were incubated with indicated concentrations of purified CHIP, with or without 10 μg/ml purified βTrCP, for 2 h at 4 °C. After extensive washing, the beads were boiled in sample buffer, and the binding was analyzed on Western blot. Signals for CHIP and GST were quantified with Odyssey software. The amount of bound CHIP was normalized against the GST signal; the amount of CHIP bound at 5 μg/ml was set at 100%. The data are representative of two independent experiments.