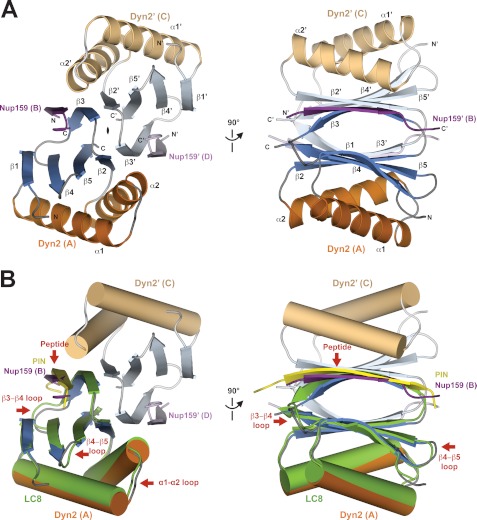
FIGURE 2.
Structure of Dyn2-Nup159 pep2 complex shows a quaternary complex composed of a Dyn2 dimer, bound to two Nup159 peptides through parallel, composite β-sheets. A, diagram of the Dyn2-Nup159 complex. Dyn2 chain A is shown in orange (α-helices) and dark blue (β-strands), and Dyn2 chain C is shown in beige (α-helices) and light blue (β-strands). The two-fold noncrystallographic symmetry operator that relates the Dyn2 and Nup159 chains in the asymmetric unit is indicated about the z axis. The image at right shows the complex after a 90° rotation about the y axis. B, the complex as shown in the two orientations in A, with Dyn2 chain A superimposed on the human dynein light chain, LC8 (light green) bound to a PIN peptide (yellow) (PDB 1CMI) after a least squares fit with an r.m.s.d. of 0.6 Å over 87 aligned Cα atoms (34). Helices are shown in cylindrical format. Structural differences between Dyn2 and human LC8 are indicated by red arrows and are dominated by loop regions as well as the bound peptides.