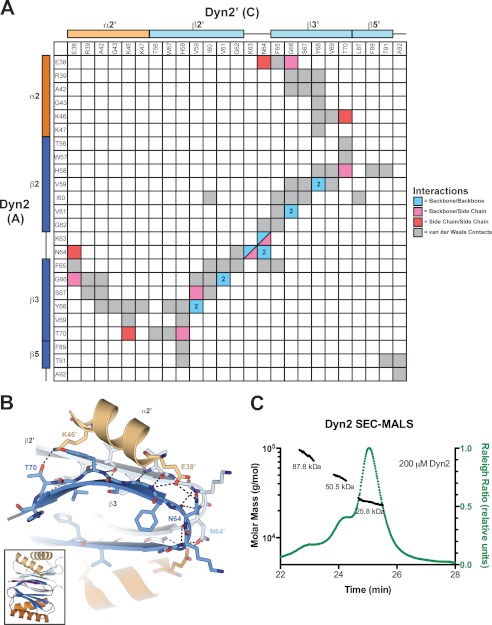
FIGURE 3.
Dyn2 homodimerizes via an extensive network of van der Waals contacts and hydrogen bonds. A, interaction matrix, showing the pseudo-symmetric bonding and contact networks formed between Dyn2 protomers A and C in the complex. Secondary structure elements corresponding to the residues of each protomer are indicated along the axes of the matrix. Backbone-backbone, backbone-side chain, side chain-side chain, and van der Waals interactions are indicated in blue, pink, red, and gray, respectively, and correlate with distances less than or equal to 3.5 (hydrogen bonds) and 4.5 Å (van der Waals contacts). Numbers in cells indicate the total number of hydrogen bonds (greater than one) between 2 residues. B, diagram of key residues and structural elements involved in the Dyn2-Dyn2 interface. The Dyn2 homodimer is shown as colored in Fig. 1. Specific Dyn2 residues mediating homodimerization are shown in stick format. Hydrogen bonds are indicated as dashed lines. The interface involves extensive antiparallel β-strand-β-strand interactions as well as contributions from the α2 helices that flank the central β-sandwich. The inset shows the relative orientation of the complex. C, size exclusion chromatography and multiangle light scattering (SEC-MALS) analysis of Dyn2, injected at an initial concentration of 200 μm (green) in 100 μl. The Raleigh Ratio elution profile was normalized. Dyn2 predominantly forms a dimer in solution at pH 6.8, with additional, higher order tetrameric and octameric species detected as well. The Dyn2 construct analyzed has a calculated monomeric molecular mass of 10,852 Da.