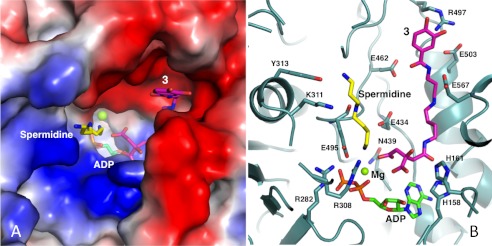
FIGURE 5.
Modeling the substrate binding pocket of AsbB. A, the binding pocket of the AsbB monomer is shown in a charge potential surface drawing with ADP (green) as well as a manually modeled spermidine (yellow) and compound 3 (pink). For the ligands, oxygen, nitrogen, and phosphorous atoms are indicated by red, blue, and orange, respectively. B, hypothetical interactions of AsbB with substrates in the active site are shown. Compound 3 is positioned so that the carboxyl end of the citrate moiety is poised to attack the α-phosphate of ATP. The N8 of spermidine is near the same phosphate, poised to attack the ester bond of adenylated 3 for formation of compound 5. Subsequent molecular modeling allows the N1-(3,4-dihydroxybenzoyl)-spermidine tail of 3 and spermidine to stretch out within the pocket between the finger and palm domain in the approximate fashion pictured. Potential protein-substrate interactions include the N1 and N8 of spermidine with Tyr-313 and Glu-459, respectively, and N1 and N4 within the spermidinyl moiety of 3 with Glu-503 and Glu-567, respectively. Residues including His-158, His-161, Arg-282, Lys-308, and Asn-439 are highly conserved among NIS synthetases (supplemental Fig. 2) and implicated in coordinating adenylation of the citryl moiety as the first step of catalysis.