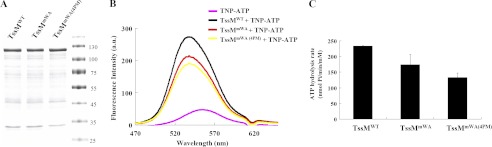
FIGURE 1.
TssM binds and hydrolyzes ATP. A, DDM-extracted membrane proteins from E. coli strain EN2 co-expressing WT or variants of TssM-His (pET-TssM-His, pET-TssMG144A/K145A-His, or pET-TssMG139A/G144A/K145A/T146A-His) with TssL (pTssL) were purified by Ni-NTA resin. The purity of wild type TssM-His (TssMWT), TssMG144A/K145A-His (TssMmWA), and TssMG139A/G144A/K145A/T146A-His (TssMmWA(4PM)) used for ATP binding and hydrolysis assays was examined by Coomassie Blue-stained SDS-PAGE. The sizes of the molecular mass standards (in kDa; Fermentas Inc.) are indicated. B, ATP binding activity of WT and its variants. Fluorescence spectra of TNP-ATP bound to WT and its variants were detected in the range of 470 to 650 nm. The spectrum for each sample is indicated as WT TssM-His (black, TssMWT +TNP-ATP), TssMG144A/K145A-His (red, TssMmWA +TNP-ATP), TssMG139A/G144A/K145A/T146A-His (yellow, TssMmWA(4PM) +TNP-ATP), or TNP-ATP alone (pink). At least three independent experiments were performed for which representative data are shown. C, ATP hydrolysis activity of WT and its variants. The ATP hydrolysis activity was determined using a malachite green assay to measure the released free inorganic phosphate upon ATP hydrolysis. Average values for ATP hydrolysis activity from three independent experiments are shown with standard deviations.