FIGURE 3.
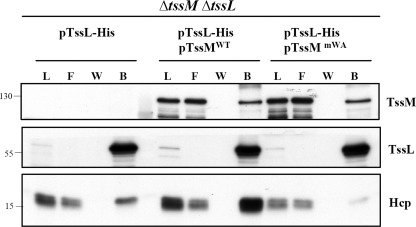
Full ATPase activity of TssM is crucial for assembly of TssM-TssL-Hcp complex. Membrane fractions isolated from the A. tumefaciens ΔtssMΔtssL double mutant expressing TssL-His alone or in the presence of WT TssM (TssMWT) or TssMG144A/K145A (TssMmWA) were solubilized with DDM. The DDM-solubilized membrane proteins were loaded onto Ni2+-NTA resin to purify TssL-His and its interacting partner, TssM. The load (L), flow-through (F) and washed (W) and bound (B) fractions were analyzed by immunoblotting with antisera against C-TssM, N-TssL, and Hcp, respectively. The sizes of the molecular mass standards (kDa) are indicated.