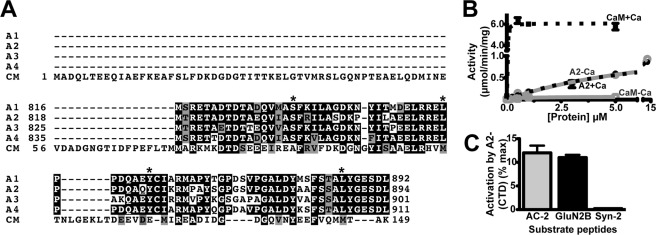
FIGURE 1.
Ca2+-independent activation of CaMKII by α-actinin-2. A, ClustalW2 alignment of the C-terminal domain (CTD) of human α-actinin-2 (A2; residues 819–894) with the CTDs of α-actinin-1, -3, and -4 (A1, A3, A4) and full-length human CaM (CM). Identical and similar residues are highlighted in black and gray, respectively. Asterisks indicate residues targeted for mutagenesis in α-actinin-2. B, concentration-dependent effects of CaM and His6-A2-CTD on CaMKII phosphorylation of autocamtide-2 in the presence of CaCl2 (+Ca; black/dashed lines) or EGTA (−Ca; gray/solid) (mean ± S.E., n = 3). C, Ca2+-independent activation of CaMKII by His6-A2-CTD (14 μm) is detected with autocamtide-2 (AC2) and GluN2B-(1290–1309), but not syntide-2. Data plotted as a percentage of maximum activation by Ca2+/CaM (mean ± S.E., n = 3).