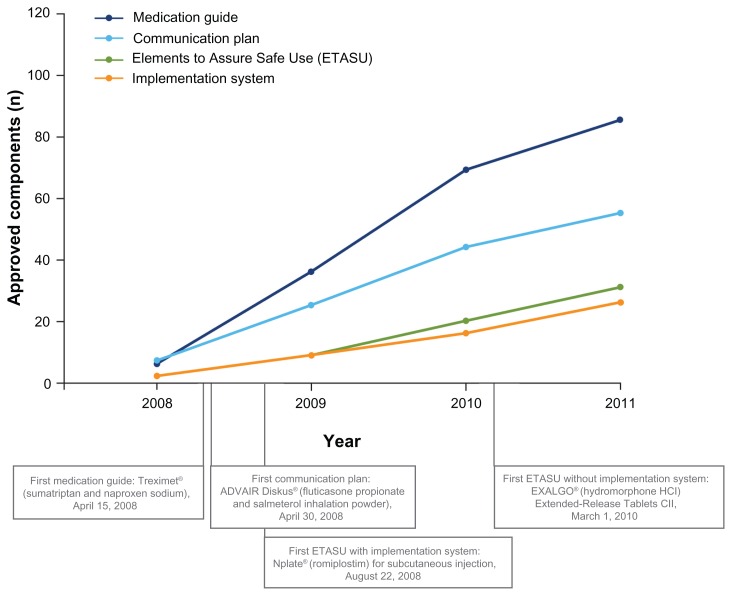
Figure 2.
Cumulative approved Risk Evaluation and Mitigation Strategy (REMS) components, 2008–2011.29
Notes: Data based on Food and Drug Administration-approved REMS (last updated December 12, 2011). All approved REMS require a timetable for submission of assessments. A total of 199 products have been approved with REMS since 2008; 81 products were subsequently released from a REMS and are not included here.29