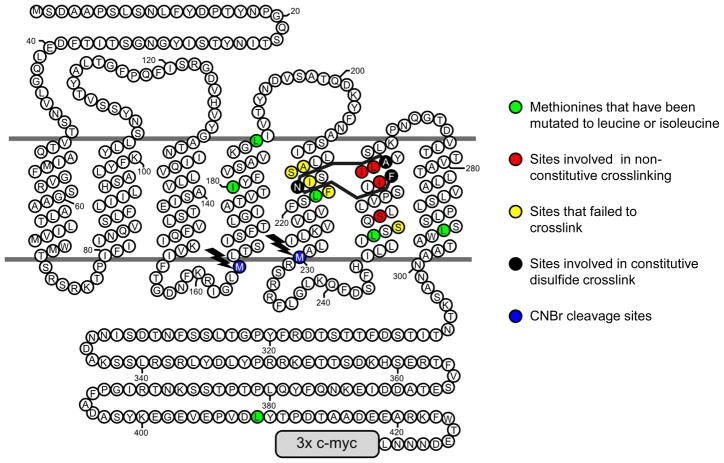
Figure 2.
Topological diagram of Ste2p showing positions of disulfides and modifications introduced to allow detection of crosslinks by immunoblotting of CNBr digests. Residues shown with green fills are naturally occurring methionines that were mutated to leucine or isoleucine to eliminate cleavage sites. Residues shown with blue fills (highlighted with lightning bolts) are remaining or introduced methionines where CNBr can cleave to create myc-tagged crosslinked or un-crosslinked fragments. The sites of disulfide bonded cysteines that result in constitutive activation are shown as residues with black fills with crosslinks indicated by solid black lines. Amino acids that, when mutated to cysteine, participate in non-constitutive crosslinks in combination with N216C are shown with red fills. Cysteine residues introduced at positions shown with yellow fills on TM5 did not form disulfide crosslinks with A265C or F262C substitutions on TM6. Cysteine introduced as a replacement for residue S252 in TM6, also shown with a yellow fill, did not form a disulfide crosslink (when mutated to cysteine) in conjunction with the N216C mutation on TM5.