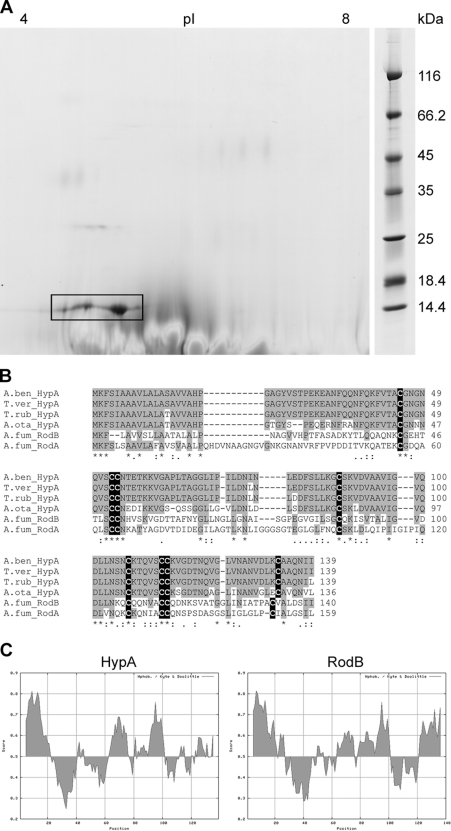
Fig 1.
The A. benhamiae hydrophobin HypA. (A) Separation of the HypA protein (ARB_06975) by two-dimensional polyacrylamide gel electrophoresis. HypA is marked by a black box. (B) Amino acid sequence comparison of A. benhamiae HypA with the homologous proteins from Trichophyton verrucosum, T. rubrum, and Arthroderma otae (Microsporum canis) and with two hydrophobins of Aspergillus fumigatus, RodA and RodB. Conserved amino acids are highlighted in gray, and cysteine residues are highlighted in black. (C) Hydropathy plot of A. benhamiae HypA and its bidirectional best hit (BBH) of A. fumigatus, RodB. Despite low sequence similarity at the amino acid level (see panel B), the distributions of hydrophilic and hydrophobic regions are similar.