Fig 4.
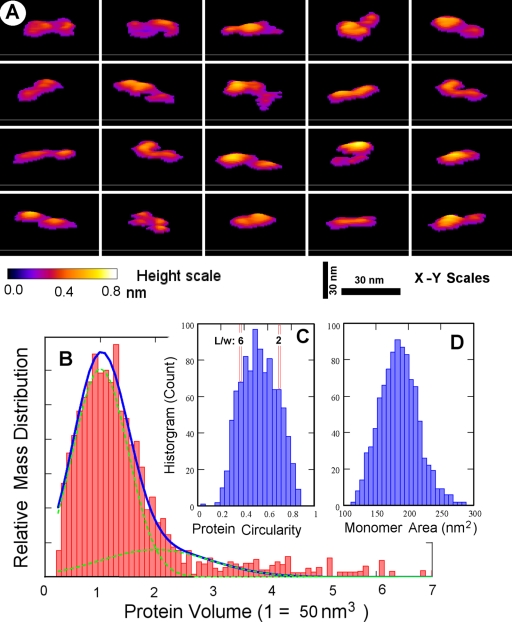
AFM shows rMTRAP as a flexible “rod”-like filamentous protein. (A) Panel of representative images, in three-dimensional plots (50-nm square viewed perpendicular to the scan direction, with a colored scale bar for height up to 0.8 nm bottom left and 30-nm bars representing the x-y scale), showing rMTRAP protein monomer shapes as seen in AFM topographies of uniformly dispersed particles on a mica surface. (B) Mass distribution histogram from ∼3,800 computed particles reveals that over 80% of rMTRAP is seen in a monomeric state (monomer-dimer decompositions illustrated under the blue curve centered at the monomer volume of 50 nm3). (C) A histogram of the circularity of these particles, defined as 4π · area/(perimeter)2, suggests flexible rod-like molecules showing twisted-ribbon-like morphologies with a typical length/width ratio of between 2 and 6. (D) A histogram of the molecular area for 955 rMTRAP monomers, having a more typical measured protein volume between 0.8 and 1.2 of the monomer value in panel B, reveals a distribution range of ∼150 to 220 nm2.