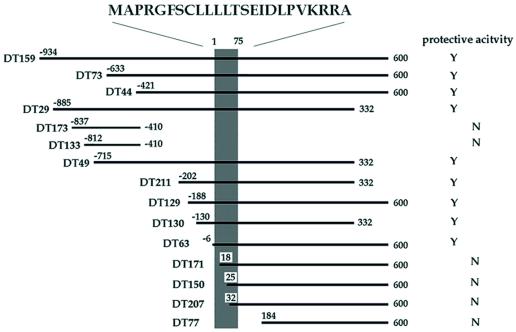
Figure 1.
Effects of DT clones on V642I-APP-induced death of neuronal cells. The region encoding the activity suppressing F11/EcR cell death by V642I-APP. The cDNA fragments are aligned within the longest clone [from −934 to 600; No. 1 base corresponds to the first base of the HN ORF (shaded area), one base before which is numbered −1], and their activities against V642I-APP-induced death of F11/EcR cells are indicated. F11/EcR cells were transfected with pIND-V642I-APP (1 μg) with 1 μg of either pEF-BOS or each cDNA fragment-coding pEF-BOS, followed by 72 h treatment with EcD, and then cell mortality was measured. When there was a statistically significant difference (P < 0.01) in cell mortality between pEF-BOS-transfected cells and each cDNA fragment-transfected cells, the protective activity was assessed to be present in the cDNA fragment and was indicated as Y; N indicates the absence of significant activity. All of the DT cDNAs with significant activity, shown as Y, caused almost complete suppression of V642I-APP-induced F11/EcR cell death. Nonprotective HN cDNAs such as DT171 were selected for the following reason: in death-trap screening, one way to augment the recovery of the plasmids from survived cells is to culture insult-treated cells for a short period and to harvest the cells at the time point when 70–80% of total cells are dead. By repeating the recovery and the retransfection, the antagonizing clones are concentrated. As in the text, we repeated this selection for a total of four rounds, picked out the final clones, and classified them into crosshybridizable groups by using dot blot hybridization with randomly selected clones. Therefore, some of the obtained clones were false positive, and each crosshybridizable group included inactive fragments, more or less.