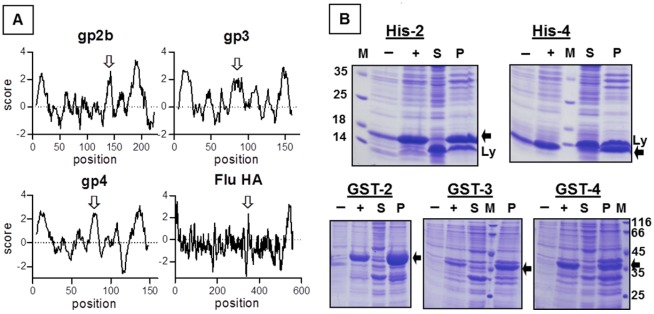
Figure 1.
Expression of the ectodomains of gp2b, gp3 and gp4 in E. coli and their accumulation in inclusion bodies. (A) Kyte-Doolittle hydropathy plots (window size 9) for gp2b, gp3, gp4 and for the hemagglutinin of influenza virus (Flu).The hydrophobicity score is plotted against the amino acid residue (position) of the respective protein. Scores above 2 are considered to be highly hydrophobic, i.e., are likely to insert into membranes. The N- and C-terminal hydrophobic regions are the proposed signal peptides and the transmembrane regions, respectively. The third hydrophobic region in the middle of the molecule, which has been shown to function as fusion peptide in the case of HA, is marked with a white arrow. The constructs used in this study contain the complete ectodomain of gp2b, gp3 and gp4, i.e., excluding the N- and C-terminal hydrophobic region. (B) Expression of 6 × His-tagged (upper panel) and Gst-EAV-proteins (lower panel) in E.coli and their accumulation in inclusion bodies. E. coli containing the respective expression plasmids were induced with IPTG and were grown overnight at room temperature. Pelleted cells were resuspended in lysis-buffer, lysed by sonication and centrifuged for 20 min at 5,000 × g. Aliquots of cell lysates before (–) and after (+) induction of protein synthesis as well as supernatants (S) and pellets (P) were subjected to SDS‑PAGE under reducing conditions and Coomassie staining. The expressed EAV‑proteins are marked with a black arrow. M: molecular mass markers as indicated, Ly: Lysozyme (14 kDa), which is added before preparation of cell lysates.