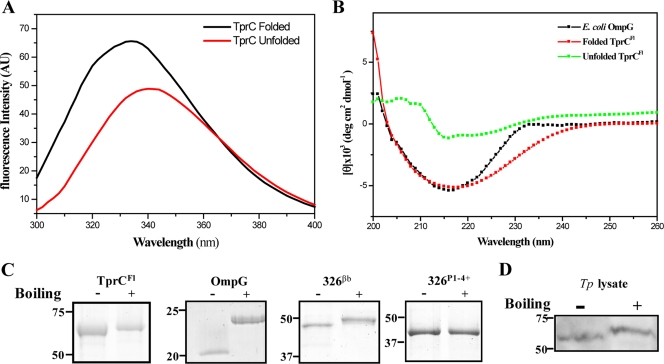
Fig 1.
TprC forms a β-barrel. (A) Tryptophan fluorescence emission spectra of unfolded and folded TprCFl in urea and DDM buffer, respectively. (B) CD spectra of unfolded and folded TprCFl (5 μM) in DDM buffer and OmpG (5 μM) in 50 mM NaCl, 10 mM Tris (pH 7.5), 0.2% n-octyl-β-d-glucopyranoside. (C) Heat modifiability of TprCFl, E. coli OmpG, 326βb, and 326P1-4+. Proteins were stained with GelCode Blue following SDS-PAGE with (+) or without (−) boiling in final sample buffer (SB). Molecular mass standards (kDa) for SDS-PAGE and immunoblot analyses are on the left of each panel. (D) T. pallidum samples (2 × 108 organisms) were dissolved in SB with (+) or without (−) boiling and immunoblotted using rat anti-TprCSp antiserum.