Abstract
Panton-Valentine leukocidin (PVL) production by methicillin-resistant Staphylococcus aureus (MRSA) was determined in vitro using the enzyme-linked immunosorbent assay (ELISA), and associations with clinical presentation and bacterial genetic characteristics were examined. PVL production ranged from 0.02 to 4.865 μg/ml and correlated with a multilocus sequence type (MLST) clonal complex associated with specific PVL phage types. A relationship between PVL production and clinical presentation or patient demographics could not be demonstrated.
TEXT
Panton-Valentine leukocidin (PVL) is a bicomponent leukotoxin that can be produced by Staphylococcus aureus, including many community-associated strains of methicillin-resistant S. aureus (CA-MRSA). The PVL-encoding genes (lukS-PV and lukF-PV) reside in the genomes of several icosahedral or elongated head-shaped temperate bacteriophages (9, 33). Clinically, MRSA which harbor PVL (PVL-MRSA) are most often associated with pyogenic skin and soft tissue infections (SSTI) (31) but can also cause life-threatening disease, most notably necrotizing pneumonia (25). Although the role of PVL as a virulence determinant has been questioned (7, 8, 14, 43), some animal-model-based investigations have demonstrated its pathogenicity (10, 13, 15, 29, 32, 42), and a clear epidemiological association is apparent between PVL and successful lineages of CA-MRSA (23; M. J. Ellington, C. Perry, M. Ganner, M. Warner, I. McCormick-Smith, R. Hill, L. Shallcross, S. Sabersheikh, A. Holmes, and A. Kearns, unpublished data). In Europe, multilocus sequence type (MLST) ST80-MRSA-SCCmecIV (European clone) predominates, but other clones, such as ST8-SCCmecIVa (USA300) and ST30-SCCmecIV (southwest Pacific clone), are notable. In England and Wales, additional PVL-MRSA MLST clonal complexes (CCs), namely, CCs 1, 5, 22, 59, and 88, and ST93 clones have also been reported (19, 21). Minor sequence variation in the PVL genes correlates with the PVL bacteriophage (3, 36), and bacteriophages are known to have limited host ranges with respect to S. aureus strains and MLST lineage (3).
Previous work has shown intra- and interstrain variation in PVL production in vitro. Interstrain variability has been previously reported for ST8 (2, 26) and ST80 and ST93 (2); ST8 PVL-positive strains corresponding to the USA300 clone have been shown to be strong PVL producers in vitro, while ST80 (European clone) strains produce 7-fold less PVL (2).
Set against this background of heterogeneity among PVL-MRSA, this study was designed to examine PVL production among diverse international lineages of PVL-MRSA identified in England and Wales and to investigate any relationships with basic bacterial genetic characteristics, including MLST CCs, PVL-encoding phages, SCCmec type (larger SCCmec clones have been shown to affect bacterial fitness [30]), and agr type (linked to the expression of virulence factors [5, 45]). Moreover, the relationship between isolates and clinical disease presentation is complex and likely to be dependent on a multiplicity of host factors. While it has been suggested previously that PVL production does not influence disease presentation in patients (2), this work was also designed to investigate whether the level of PVL production may be related to clinical presentation and to analyze patient demographics and assess epidemiological relationships with respect to clinical disease.
The Staphylococcus Reference Unit (SRU) for England and Wales receives isolates from a wide spectrum of disease presentations for surveillance and outbreak investigation purposes. In this work, 142 isolates were studied; these had been referred to the SRU between 2005 and 2008 (from a total prevalence of 1,477 PVL-MRSA) from centers across the nine regions of England designated by the Health Protection Agency (http://www.hpa.org.uk/HPA/ProductsServices/InfectiousDiseases/RegionalMicrobiologyNetwork/) and from Wales (defined as a 10th region for the purposes of this study) and were selected to maximize demographic, phenotypic, and genotypic diversity. The study isolates were selected to represent the main lineages of PVL-MRSA occurring nationally; all had been characterized previously by MLST, pulsed-field gel electrophoresis (PFGE), SCCmec, spa, and arginine-catabolic mobile element (ACME) PCR as belonging to MLST CCs 1, 5, 8, 22, 30, 59, 80, and 88 and ST93 (4, 19–21, 22). Where possible, isolates were also selected to include a range of disease presentations, which were categorized into six groups: community-acquired pneumonia (CAP), bacteremia (Bact), SSTI, upper respiratory tract infection (URTI), asymptomatic carriage (AS), and not known (NK).
Eight PCRs were performed to detect five of the PVL-encoding phages (ΦSa2958, ΦSa2MW, ΦPVL, Φ108PVL, and ΦSLT), as described previously (4, 34). A PCR (fragment size, 680 bp) was designed to detect a sixth PVL phage, ΦSa2USA, using methods and primers described by Boakes et al. (3) (the more recently described Φtp310-1 was not examined [46]). Study isolates were cultured in triplicate in casein hydrolysate-yeast(CCY) medium, and PVL was detected and quantified using an antibody sandwich enzyme-linked immunosorbent assay (ELISA) targeting LukS-PV as described by Badiou et al. (2). The mean PVL production from triplicate cultures was taken for each isolate.
The Kruskal-Wallis equality-of-populations rank test was used as an initial test for variation in PVL production between isolates. A generalized linear model (GLM) was used to examine how PVL production was affected by a number of different fixed, specific variables, including bacterial lineage (MLST CC), type of PVL-encoding phage, and SCCmec and agr type. Possible interaction between these variables was also explored. In addition, the effect of PVL production on the disease presentation was examined. The data were normalized by transforming the PVL production data by log (PVL values × 100) (to allow for low values in the data set). A minimally adequate model was derived in order to best describe the relationship between the variables and PVL production. Categorical scatter plots showing the mean and 95% confidence intervals were used to identify differences in PVL titers across different variables. The level of statistical significance was set at a P of <0.05. Contingency tables were created, and Pearson's chi-square tests were used to examine the significance of age, sex of the patient, and geographic region on clinical disease presentation. Statistical analysis and plotting were performed in R (40).
This work shows a hitherto-unrecognized variability in the production of PVL toxin across internationally disseminated lineages of PVL-MRSA that have emerged recently. Using ELISA, we determined that the PVL-MRSA tested (chosen to be representative of genetically diverse strains identified in England and Wales [n = 142]) produced 0.02 to 4.865 μg/ml PVL (mean, 0.5 μg/ml) (see Table S1 in the supplemental material). Furthermore, 94% (n = 134) of study isolates produced enough PVL in vitro to induce human polymorphonuclear leukocyte activation (>0.05 μg/ml [28]) and 60% (n = 85) produced PVL at concentrations toxic for human leukocytes (>0.3 μg [24]). These data support previous observations that PVL may induce a host inflammatory response during infection (1, 2).
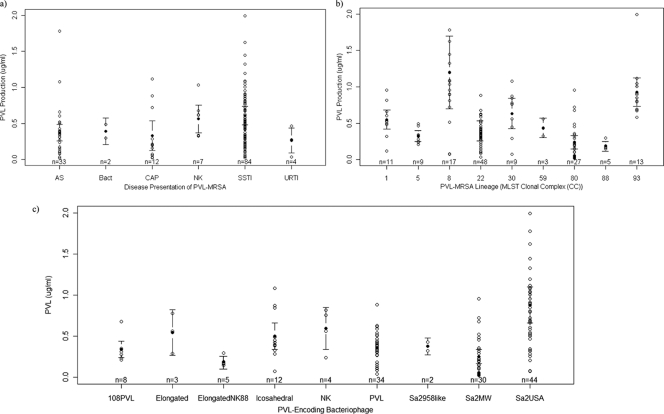
Patient demographics and clinical presentations for the 142 individuals were typical for PVL-MRSA (27), and cases were distributed widely throughout England and Wales (see Table S1 in the supplemental material). Epidemiological data show that PVL-positive S. aureus strains are associated with some severe forms of skin infection (12, 31, 37, 47), bone and joint infection (3, 9), and necrotizing pneumonia (12, 13). For these infections, experimental data support the role of PVL in disease severity (6, 10, 13, 41). However, in this study, there was no correlation between clinical presentation and high levels of PVL production observed in vitro (P > 0.05) (Fig. 1a), which may be attributable to the heterogeneous nature of the infections and patients. Age, sex, and geographic region did not have a significant effect on disease presentation (P > 0.05) (see Table S2 in the supplemental material). For those infected with PVL-MRSA, it is probable that bacterial and host factors, such as bacterial load, body site, nature of the infection, history of recurrent disease, therapeutic strategies, underlying comorbidities, and immune status, influence the level of PVL produced during the course of infection (11). Due to the complexity of virulence and the multifactorial nature of pathogenicity in PVL-MRSA infection, it is likely that no single bacterial virulence factor is responsible for causing severe infection. Whether PVL is a marker for disease severity or not, it seems likely that antibiotic combinations that inhibit both bacterial replication and the release of virulence factors, including PVL, may improve the outcome of severe infections caused by PVL-producing S. aureus strains (in vitro, β-lactam agents increase PVL release, while protein synthesis inhibitors, such as clindamycin, rifampin, and linezolid, suppress it [16, 17]).
Fig 1.
Categorical scatter plots of mean PVL concentrations (μg/ml) produced by PVL-MRSA from different disease presentations (a), MLST CCs (b), and PVL-encoding phages (c). AS, asymptomatic carriage, CAP, community-acquired pneumonia; SSTI, skin and soft tissue infection; URTI, upper respiratory tract infection; Bact, bacteremia; NK, not known. The number of cases for each disease presentation is shown (n). Black dots indicate means, and the error bars show the 95% confidence intervals surrounding the means. PVL production was measured in μg/ml. The isolate producing 4.86 μg/ml was considered an outlier and is not shown.
These data support previous evidence that within MLST lineages, isolates harbor different PVL phages (3) and produce variable amounts of PVL (see Table S1 in the supplemental material and Fig. 1b and c). Little variation was observed within the replicates of each isolate (standard deviation [SD] range, 0.062 to 0.206) but intralineage variation in PVL production did occur, evidenced by the broad differences in standard deviations shown in Table S1 (e.g., CC8). Using the Kruskal-Wallis equality-of-populations rank test, a marked difference in PVL production across the study isolates was apparent (P < 0.001). Significant variation between the lineages of PVL-MRSA was identified; CC8 with ΦSa2USA produced the most PVL, significantly more than CCs 5, 22, 80, and 88 (P value < 0.001) (Tables S1 and S3 and Fig. 1b). CC88 with an unknown elongated PVL phage produced the smallest amount of PVL, significantly less than all other lineages excepting CC80 (P < 0.05) (Tables S1 and S3 and Fig. 1b). The GLM showed a significant relationship between PVL production and MLST (CC) (P < 0.001) (Table S3). Previous work has shown that the insertion of PVL-encoding phage is lineage specific (3, 45) and that expression of the PVL genes is dependent on phage life cycle (45) and host chromosomal regulatory networks; notably, agr and saeRS are linked to the pathogenesis of CA-MRSA (5, 35, 44, 45). This study shows that agr type (or SCCmec type) did not significantly affect PVL production; however, differences attributable to variable agr expression cannot be ruled out (39).
It is important to acknowledge that these in vitro observations may not be representative of PVL production in vivo (2). Some parallels may exist, however, as recent work has shown that PVL production in vivo is increased by β-lactam treatment (18). Reliable comparison of in vitro and in vivo findings would entail quantitative cultures of clinical material to estimate bacterial loads, which is difficult to correlate (26) and will likely be further compounded by difficulties in correlating PVL level with clinical disease, due to the complex and obscuring effect of host factors.
In summary, this work shows that PVL production in MRSA is variable, with significant association with MLST CC. Specificities of PVL-encoding phages for MLST CCs were also apparent (3). PVL production was not affected by SCCmec or agr type, although the effect of agr expression on PVL production warrants further investigation. While further work probing the relationship between PVL production and disease outcome is necessary, this work suggests that there is no statistical relationship between PVL production and the most severe clinical presentations of PVL-MRSA infection. In view of the complex nature of pathogenicity, adapting therapeutic strategies in accordance with current guidance (38) may ameliorate disease severity.
(Parts of this work were presented at the 20th European Congress of Clinical Microbiology and Infectious Disease [ECCMID], Vienna, Austria, 10 to 13 April 2010, and the 14th International Symposium on Staphylococci and Staphylococcal Infections [ISSSI], Bath, United Kingdom, 6 to 9 September 2010.)
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to C. Ratat and M. Leportier for their technical assistance and to bioMérieux for providing the PVL ELISA. We thank Peter Staves for Bioinformatics and statistical support and Marjorie Ganner for technical assistance.
This work was funded by the Health Protection Agency, United Kingdom.
We have no conflicts of interest.
Footnotes
Published ahead of print 28 December 2011
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1. Badiou C, et al. 2008. Panton-Valentine leukocidin is expressed at toxic levels in human skin abscesses. Clin. Microbiol. Infect. 14:1180–1183 [DOI] [PubMed] [Google Scholar]
- 2. Badiou C, et al. 2010. Rapid detection of Staphylococcus aureus Panton-Valentine leukocidin by enzyme-linked immunosorbent assay and immunochromatographic tests in clinical specimens. J. Clin. Microbiol. 48:1384–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boakes E, et al. 2011. Distinct bacteriophages encoding PVL among international clones of PVL-MRSA. J. Clin. Microbiol. 49:684–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boakes E, et al. 2011. Molecular diversity within clonal complex 22 methicillin-resistant Staphylococcus aureus encoding Panton-Valentine leukocidin in England and Wales. Clin. Microbiol. Infect. 17:140–145 [DOI] [PubMed] [Google Scholar]
- 5. Bronner S, Stoessel P, Gravet A, Monteil H, Prevost G. 2000. Variable expressions of Staphylococcus aureus bicomponent leucotoxins semiquantified by competitive reverse transcription-PCR. Appl. Environ. Microbiol. 66:3931–3938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown EL, et al. 2009. The Panton-Valentine leukocidin vaccine protects mice against lung and skin infections caused by Staphylococcus aureus USA300. Clin. Microbiol. Infect. 15:156–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bubeck Wardenburg J, Bae T, Otto M, DeLeo FR, Schneewind O. 2007. Poring over pores: alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat. Med. 13:1405–1406 [DOI] [PubMed] [Google Scholar]
- 8. Bubeck Wardenburg J, Palazzolo-Ballance AM, Otto M, Schneewind O, DeLeo FR. 2008. Panton-Valentine leukocidin is not a virulence determinant in murine models of community-associated methicillin-resistant Staphylococcus aureus disease. J. Infect. Dis. 198:1166–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Canchaya C, Proux C, Fournous G, Bruttin A, Brussow H. 2003. Prophage genomics. Microbiol. Mol. Biol. Rev. 67:238–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cremieux AC, et al. 2009. Panton-Valentine leukocidin enhances the severity of community-associated methicillin-resistant Staphylococcus aureus rabbit osteomyelitis. PLoS One 4:e7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Croze M, et al. 2009. Serum antibodies against Panton-Valentine leukocidin in a normal population and during Staphylococcus aureus infection. Clin. Microbiol. Infect. 15:144–148 [DOI] [PubMed] [Google Scholar]
- 12. Daskalaki M, et al. 2010. Panton-Valentine leukocidin-positive Staphylococcus aureus skin and soft tissue infections among children in an emergency department in Madrid, Spain. Clin. Microbiol. Infect. 16:74–77 [DOI] [PubMed] [Google Scholar]
- 13. Diep BA, et al. 2010. Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury. Proc. Natl. Acad. Sci. U. S. A. 107:5587–5592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Diep BA, Otto M. 2008. The role of virulence determinants in community-associated MRSA pathogenesis. Trends Microbiol. 16:361–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Diep BA, et al. 2008. Contribution of Panton-Valentine leukocidin in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. PLoS One 3:e3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dumitrescu O, et al. 2008. Effect of antibiotics, alone and in combination, on Panton-Valentine leukocidin production by a Staphylococcus aureus reference strain. Clin. Microbiol. Infect. 14:384–388 [DOI] [PubMed] [Google Scholar]
- 17. Dumitrescu O, et al. 2007. Effect of antibiotics on Staphylococcus aureus producing Panton-Valentine leukocidin. Antimicrob. Agents Chemother. 51:1515–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dumitrescu O, et al. 2011. Beta-lactams interfering with PBP1 induce Panton-Valentine leukocidin expression by triggering sarA and rot global regulators of Staphylococcus aureus. Antimicrob. Agents Chemother. 55:3261–3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ellington MJ, Ganner M, Warner M, Cookson BD, Kearns AM. 2010. Polyclonal multiply antibiotic-resistant methicillin-resistant Staphylococcus aureus with Panton-Valentine leucocidin in England. J. Antimicrob. Chemother. 65:46–50 [DOI] [PubMed] [Google Scholar]
- 20. Ellington MJ, et al. 2007. Is Panton-Valentine leucocidin associated with the pathogenesis of Staphylococcus aureus bacteraemia in the UK? J. Antimicrob. Chemother. 60:402–405 [DOI] [PubMed] [Google Scholar]
- 21. Ellington MJ, et al. 2009. Clinical and molecular epidemiology of ciprofloxacin-susceptible MRSA encoding PVL in England and Wales. Eur. J. Clin. Microbiol. Infect. Dis. 28:1113–1121 [DOI] [PubMed] [Google Scholar]
- 22. Ellington MJ, Yearwood L, Ganner M, East C, Kearns AM. 2008. Distribution of the ACME-arcA gene among methicillin-resistant Staphylococcus aureus from England and Wales. J. Antimicrob. Chemother. 61:73–77 [DOI] [PubMed] [Google Scholar]
- 23. Etienne J. 2005. Panton-Valentine leukocidin: a marker of severity for Staphylococcus aureus infection? Clin. Infect. Dis. 41:591–593 [DOI] [PubMed] [Google Scholar]
- 24. Genestier AL, et al. 2005. Staphylococcus aureus Panton-Valentine leukocidin directly targets mitochondria and induces Bax-independent apoptosis of human neutrophils. J. Clin. Invest. 115:3117–3127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gillet Y, et al. 2002. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet 359:753–759 [DOI] [PubMed] [Google Scholar]
- 26. Hamilton SM, et al. 2007. In vitro production of Panton-Valentine leukocidin among strains of methicillin-resistant Staphylococcus aureus causing diverse infections. Clin. Infect. Dis. 45:1550–1558 [DOI] [PubMed] [Google Scholar]
- 27. Herold BC, et al. 1998. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA 279:593–598 [DOI] [PubMed] [Google Scholar]
- 28. Konig B, Prevost G, Piemont Y, Konig W. 1995. Effects of Staphylococcus aureus leukocidins on inflammatory mediator release from human granulocytes. J. Infect. Dis. 171:607–613 [DOI] [PubMed] [Google Scholar]
- 29. Labandeira-Rey M, et al. 2007. Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science 315:1130–1133 [DOI] [PubMed] [Google Scholar]
- 30. Lee SM, et al. 2007. Fitness cost of staphylococcal cassette chromosome mec in methicillin-resistant Staphylococcus aureus by way of continuous culture. Antimicrob. Agents Chemother. 51:1497–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lina G, et al. 1999. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin. Infect. Dis. 29:1128–1132 [DOI] [PubMed] [Google Scholar]
- 32. Lipinska U, et al. 2011. Panton-Valentine leukocidin does play a role in the early stage of Staphylococcus aureus skin infections: a rabbit model. PLoS One 6:e22864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ma XX, Ito T, Chongtrakool P, Hiramatsu K. 2006. Predominance of clones carrying Panton-Valentine leukocidin genes among methicillin-resistant Staphylococcus aureus strains isolated in Japanese hospitals from 1979 to 1985. J. Clin. Microbiol. 44:4515–4527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ma XX, et al. 2008. Two different Panton-Valentine leukocidin phage lineages predominate in Japan. J. Clin. Microbiol. 46:3246–3258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Montgomery CP, Boyle-Vavra S, Daum RS. 2010. Importance of the global regulators Agr and SaeRS in the pathogenesis of CA-MRSA USA300 infection. PLoS One 5:e15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Otter JA, Kearns AM, French GL, Ellington MJ. 2010. Panton-Valentine leukocidin-encoding bacteriophage and gene sequence variation in community-associated methicillin-resistant Staphylococcus aureus. Clin. Microbiol. Infect. 16:68–73 [DOI] [PubMed] [Google Scholar]
- 37. Prevost G, et al. 1995. Epidemiological data on Staphylococcus aureus strains producing synergohymenotropic toxins. J. Med. Microbiol. 42:237–245 [DOI] [PubMed] [Google Scholar]
- 38. PVL Sub-group of the Steering Group on Healthcare Associated Infection 7 November 2008. Guidance on the diagnosis and management of PVL-associated Staphylococcus aureus infections (PVL-SA) in England, 2nd ed Health Protection Agency, London, United Kingdom [Google Scholar]
- 39. Queck SY, et al. 2008. RNAIII-independent target gene control by the agr quorum-sensing system: insight into the evolution of virulence regulation in Staphylococcus aureus. Mol. Cell 32:150–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. The R Development Core Team 2008. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria [Google Scholar]
- 41. Tseng CW, et al. 2009. Staphylococcus aureus Panton-Valentine leukocidin contributes to inflammation and muscle tissue injury. PLoS One 4:e6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Varshney AK, et al. 2010. Augmented production of Panton-Valentine leukocidin toxin in methicillin-resistant and methicillin-susceptible Staphylococcus aureus is associated with worse outcome in a murine skin infection model. J. Infect. Dis. 201:92–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Voyich JM, et al. 2006. Is Panton-Valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? J. Infect. Dis. 194:1761–1770 [DOI] [PubMed] [Google Scholar]
- 44. Voyich JM, et al. 2009. The saeR/S gene regulatory system is essential for innate immune evasion by Staphylococcus aureus. J. Infect. Dis. 199:1698–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wirtz C, Witte W, Wolz C, Goerke C. 2009. Transcription of the phage-encoded Panton-Valentine leukocidin of Staphylococcus aureus is dependent on the phage life-cycle and on the host background. Microbiology 155:3491–3499 [DOI] [PubMed] [Google Scholar]
- 46. Wirtz C, Witte W, Wolz C, Goerke C. 2010. Insertion of host DNA into PVL-encoding phages of the Staphylococcus aureus lineage ST80 by intra-chromosomal recombination. Virology 406:322–327 [DOI] [PubMed] [Google Scholar]
- 47. Yamasaki O, et al. 2005. The association between Staphylococcus aureus strains carrying Panton-Valentine leukocidin genes and the development of deep-seated follicular infection. Clin. Infect. Dis. 40:381–385 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.