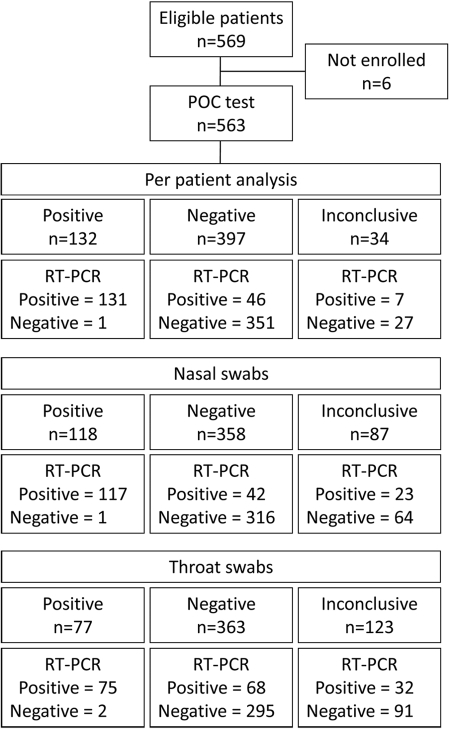
Fig 1.
Flow diagram of the clinical validation of a point-of-care test compared to RT-PCR for influenza virus. Each patient had a nasal swab and a throat swab specimen taken. The diagram shows results when analyzed per patient and for nasal and throat swabs separately. In the per patient analysis, a patient was considered positive if the nasal swab or the throat swab (or both) was positive for influenza virus (A or B). Patients were treated as negative if both the nasal and throat swabs were negative for influenza virus (A and B) or—for the POC test—if one swab specimen was negative and the other was inconclusive.