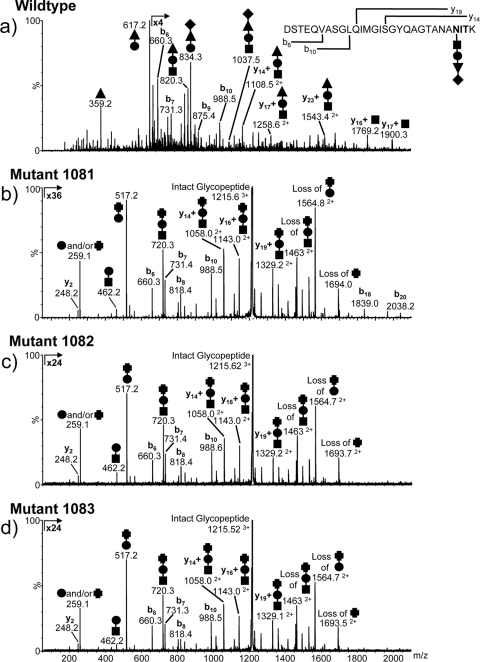
Fig 4.
Nano-LC–MS/MS analysis of the FlaB2 tryptic glycopeptide T53–81 from the wild-type (a) and mmp1081 (b), mmp1082 (c), and mmp1083 (d) mutant strains of M. maripaludis. The major carbohydrate oxonium ions are identified in the MS/MS spectra by using symbols to indicate the sugar residues present [■, GalNAc; ●, GlcNAc3NAcA; ▲, ManNAc3NAmA6Thr; ♦, (5S)-2-acetamido-2,4-dideoxy-5-O-methyl-α-l-erythro-hexos-5-ulo-1,5-pyranose; and ✚, ManNAc3NAcA]. The b and y ions arising from fragmentation of the peptide bonds are also shown. The glycopeptide from the mmp1081 mutant strain (b) was modified with a trisaccharide composed of the linking GalNAc (■), the GlcNAc3NacA (●), and a terminal sugar that is likely to be di-N-acetyl-mannuronic acid (ManNAc3NAcA [✚). In normal media, both the mmp1082 and mmp1083 mutant strains expressed predominantly the wild-type glycan. However, in nitrogen-free medium supplemented with alanine, both strains expressed predominantly the Δmmp1081 glycan modification (c and d).