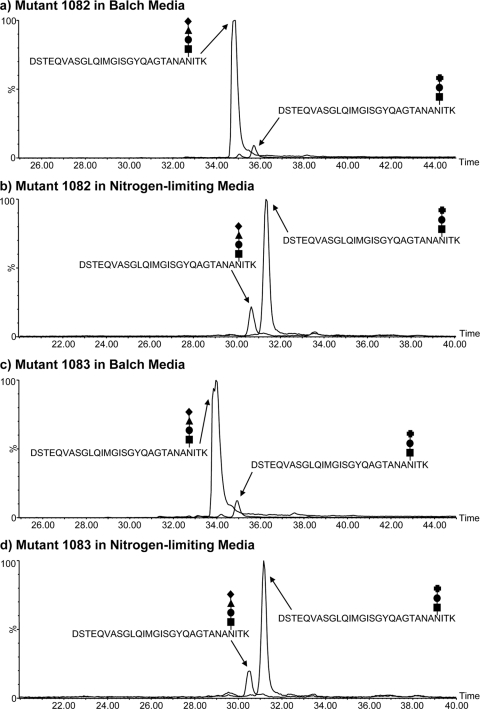
Fig 5.
Extracted ion chromatograms (EICs) of the wild-type and mutant glycan versions of the FlaB2 tryptic glycopeptide T53–81 isolated from the mmp1082 and mmp1083 mutant strains grown in Balch medium and under ammonia-limiting conditions. The EICs for triply protonated ions for the wild-type and mutant T53–81 glycopeptides are plotted together for the mmp1082 strain grown in Balch (a) and nitrogen-limiting (b) media. The same ions are shown for the mmp1083 strain (c and d). The relative peak areas (expressed as percentages) of the two glycopeptide ions in all the strains examined in this study are presented in Table 2. The glycan symbols used here are explained in the legend for Fig. 4. Though both peptide glycoforms were expressed in the Δmmp1082 and Δmmp1083 strains under the two conditions, the wild-type glycopeptide predominated (>90%) in the Balch-grown strains, while the reverse was true under ammonia-limiting conditions.