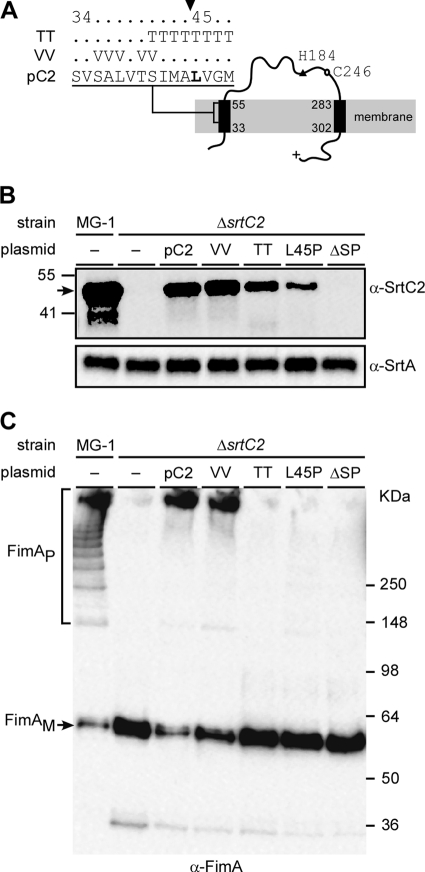
Fig 1.
Requirements for fimbrial polymerization of the SrtC2 N-terminal signal peptide sequence. Predicted membrane topology of the type 2 fimbria-specific sortase SrtC2 is shown in panel A, with the N terminus possessing a signal peptide sequence. The filled arrowhead specifies a cleavage site. Threonin (TT) and valine (VV) substitutions of this cleavage site are indicated. Catalytic residues H184 and C246 are located upstream of the C-terminal transmembrane helix. (B and C) Cell wall and membrane fractions were harvested from Actinomyces cells of the wild-type MG-1 strain and its isogenic derivative strains. Equivalent protein samples were separated on 4 to 12% Tris-glycine gradient gel and detected by immunoblotting with antibodies against SrtC2 and SrtA (α-SrtC2 and α-SrtA; membrane fractions) (B) or FimA (α-FimA; cell wall fractions) (C). The positions of high-molecular-mass polymers (FimAP) and monomers (FimAM) and molecular mass markers are indicated.