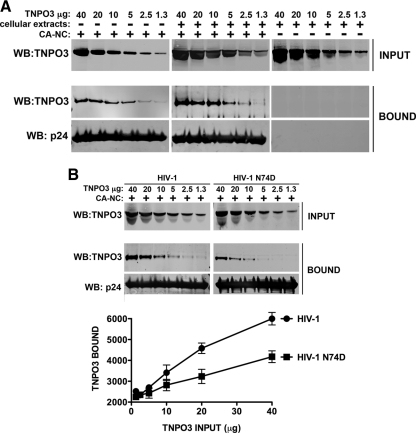
Fig 2.
Binding of TNPO3 to HIV-1 capsid-nucleocapsid complexes. (A) The indicated amounts of recombinant TNPO3 protein were incubated with HIV-1 CA-NC complexes at room temperature for 1 h in the presence of cellular extracts or capsid-binding buffer. The mixtures were applied onto a 70% sucrose cushion and centrifuged. INPUT represents the mixtures analyzed by Western blotting before being applied to the 70% cushion. The INPUT mixtures were Western blotted using anti-TNPO3 antibodies. The pellet from the 70% cushion (BOUND) was analyzed by Western blotting using anti-TNPO3 and anti-capsid antibodies. To demonstrate that TNPO3 is unable to traverse the cushion by itself, we applied the different amounts of TNPO3 incubated in cellular extracts without HIV-1 CA-NC complexes onto a 70% sucrose cushion. The results of three independent experiments were similar; the result of a single experiment is shown. (B) Similar amounts of recombinant TNPO3 were incubated with HIV-1 wt or mutant CA-NC complexes for 1 h in the presence of cellular extracts. As described above, input and bound fractions were analyzed by fluorescent Western blotting using anti-TNPO3 and anti-capsid antibodies. Quantifications of TNPO3 bound versus TNPO3 input fractions of three independent experiments were plotted with their respective standard deviations on the lower panel. Statistical differences were given as P values of <0.001 for an input fraction of 40 μg by two-way ANOVA followed by the Bonferroni post test.