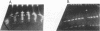Abstract
Large circular amplified DNAs (30 and 85 kb) present in methotrexate-resistant Leishmania major appear to migrate anomalously in pulsed field-gradient electrophoresis (PFGE), exhibiting pulse time-dependent mobility and migrating along a different apparent path relative to the large linear chromosomal DNAs. Quantitative studies indicate that the relative pulse-time dependence is actually conferred by the mobility properties of the large linear DNAs. One contributing factor to the difference in migration path is variability in the intrinsic voltage-dependence of mobility of supercoiled and linear DNAs, in combination with the asymmetrical/inhomogeneous voltage gradients. Certain linear chromosomes exhibit a previously undescribed pulse-time dependence in the voltage-dependence of mobility. When enzymatically relaxed or physically nicked the large circular DNAs fail to leave the well using any pulse time, a property also observed in conventional electrophoresis. These findings are relevant to PFGE theory, and its application to the study of circular DNA amplification in Leishmania and other species.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beverley S. M., Coderre J. A., Santi D. V., Schimke R. T. Unstable DNA amplifications in methotrexate-resistant Leishmania consist of extrachromosomal circles which relocalize during stabilization. Cell. 1984 Sep;38(2):431–439. doi: 10.1016/0092-8674(84)90498-7. [DOI] [PubMed] [Google Scholar]
- Beverley S. M., Ellenberger T. E., Cordingley J. S. Primary structure of the gene encoding the bifunctional dihydrofolate reductase-thymidylate synthase of Leishmania major. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2584–2588. doi: 10.1073/pnas.83.8.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverley S. M., Ismach R. B., Pratt D. M. Evolution of the genus Leishmania as revealed by comparisons of nuclear DNA restriction fragment patterns. Proc Natl Acad Sci U S A. 1987 Jan;84(2):484–488. doi: 10.1073/pnas.84.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carle G. F., Frank M., Olson M. V. Electrophoretic separations of large DNA molecules by periodic inversion of the electric field. Science. 1986 Apr 4;232(4746):65–68. doi: 10.1126/science.3952500. [DOI] [PubMed] [Google Scholar]
- Carle G. F., Olson M. V. An electrophoretic karyotype for yeast. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3756–3760. doi: 10.1073/pnas.82.11.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carle G. F., Olson M. V. Separation of chromosomal DNA molecules from yeast by orthogonal-field-alternation gel electrophoresis. Nucleic Acids Res. 1984 Jul 25;12(14):5647–5664. doi: 10.1093/nar/12.14.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G., Vollrath D., Davis R. W. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science. 1986 Dec 19;234(4783):1582–1585. doi: 10.1126/science.3538420. [DOI] [PubMed] [Google Scholar]
- Coderre J. A., Beverley S. M., Schimke R. T., Santi D. V. Overproduction of a bifunctional thymidylate synthetase-dihydrofolate reductase and DNA amplification in methotrexate-resistant Leishmania tropica. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2132–2136. doi: 10.1073/pnas.80.8.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comeau A. M., Miller S. I., Wirth D. F. Chromosome location of four genes in Leishmania. Mol Biochem Parasitol. 1986 Nov;21(2):161–169. doi: 10.1016/0166-6851(86)90019-8. [DOI] [PubMed] [Google Scholar]
- Ebrahimzadeh A., Jones T. C. A comparative study of different Leishmania tropica isolates from Iran: correlation between infectivity and cytochemical properties. Am J Trop Med Hyg. 1983 Jul;32(4):694–702. doi: 10.4269/ajtmh.1983.32.694. [DOI] [PubMed] [Google Scholar]
- Fisher M. P., Dingman C. W. Role of molecular conformation in determining the electrophoretic properties of polynucleotides in agarose-acrylamide composite gels. Biochemistry. 1971 May 11;10(10):1895–1899. doi: 10.1021/bi00786a026. [DOI] [PubMed] [Google Scholar]
- Gardiner K., Laas W., Patterson D. Fractionation of large mammalian DNA restriction fragments using vertical pulsed-field gradient gel electrophoresis. Somat Cell Mol Genet. 1986 Mar;12(2):185–195. doi: 10.1007/BF01560665. [DOI] [PubMed] [Google Scholar]
- Garvey E. P., Coderre J. A., Santi D. V. Selection and properties of Leishmania tropica resistant to 10-propargyl-5,8-dideazafolate, an inhibitor of thymidylate synthetase. Mol Biochem Parasitol. 1985 Oct;17(1):79–91. doi: 10.1016/0166-6851(85)90129-x. [DOI] [PubMed] [Google Scholar]
- Garvey E. P., Santi D. V. Stable amplified DNA in drug-resistant Leishmania exists as extrachromosomal circles. Science. 1986 Aug 1;233(4763):535–540. doi: 10.1126/science.3726545. [DOI] [PubMed] [Google Scholar]
- Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- Giannini S. H., Schittini M., Keithly J. S., Warburton P. W., Cantor C. R., Van der Ploeg L. H. Karyotype analysis of Leishmania species and its use in classification and clinical diagnosis. Science. 1986 May 9;232(4751):762–765. doi: 10.1126/science.3961502. [DOI] [PubMed] [Google Scholar]
- Grumont R., Washtien W. L., Caput D., Santi D. V. Bifunctional thymidylate synthase-dihydrofolate reductase from Leishmania tropica: sequence homology with the corresponding monofunctional proteins. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5387–5391. doi: 10.1073/pnas.83.15.5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hightower R. C., Metge D. W., Santi D. V. Plasmid migration using orthogonal-field-alternation gel electrophoresis. Nucleic Acids Res. 1987 Oct 26;15(20):8387–8398. doi: 10.1093/nar/15.20.8387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. H., Grossman L. I. Electrophoresis of DNA in agarose gels. Optimizing separations of conformational isomers of double- and single-stranded DNAs. Biochemistry. 1977 Sep 20;16(19):4217–4225. doi: 10.1021/bi00638a014. [DOI] [PubMed] [Google Scholar]
- Levene S. D., Zimm B. H. Separations of open-circular DNA using pulsed-field electrophoresis. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4054–4057. doi: 10.1073/pnas.84.12.4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickel S., Arena V., Jr, Bauer W. Physical properties and gel electrophoresis behavior of R12-derived plasmid DNAs. Nucleic Acids Res. 1977;4(5):1465–1482. doi: 10.1093/nar/4.5.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush M. G., Misra R. Extrachromosomal DNA in eucaryotes. Plasmid. 1985 Nov;14(3):177–191. doi: 10.1016/0147-619x(85)90001-0. [DOI] [PubMed] [Google Scholar]
- Schimke R. T. Gene amplification in cultured animal cells. Cell. 1984 Jul;37(3):705–713. doi: 10.1016/0092-8674(84)90406-9. [DOI] [PubMed] [Google Scholar]
- Schindler C. W., Krolewski J. J., Rush M. G. Selective trapping of circular double-stranded DNA molecules in solidifying agarose. Plasmid. 1982 May;7(3):263–270. doi: 10.1016/0147-619x(82)90007-5. [DOI] [PubMed] [Google Scholar]
- Scholler J. K., Reed S. G., Stuart K. Molecular karyotype of species and subspecies of Leishmania. Mol Biochem Parasitol. 1986 Sep;20(3):279–293. doi: 10.1016/0166-6851(86)90108-8. [DOI] [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Serwer P., Allen J. L. Conformation of double-stranded DNA during agarose gel electrophoresis: fractionation of linear and circular molecules with molecular weights between 3 X 10(6) and 26 X 10(6). Biochemistry. 1984 Feb 28;23(5):922–927. doi: 10.1021/bi00300a020. [DOI] [PubMed] [Google Scholar]
- Spithill T. W., Samaras N. The molecular karyotype of Leishmania major and mapping of alpha and beta tubulin gene families to multiple unlinked chromosomal loci. Nucleic Acids Res. 1985 Jun 11;13(11):4155–4169. doi: 10.1093/nar/13.11.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark G. R., Wahl G. M. Gene amplification. Annu Rev Biochem. 1984;53:447–491. doi: 10.1146/annurev.bi.53.070184.002311. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Schwartz D. C., Cantor C. R., Borst P. Antigenic variation in Trypanosoma brucei analyzed by electrophoretic separation of chromosome-sized DNA molecules. Cell. 1984 May;37(1):77–84. doi: 10.1016/0092-8674(84)90302-7. [DOI] [PubMed] [Google Scholar]