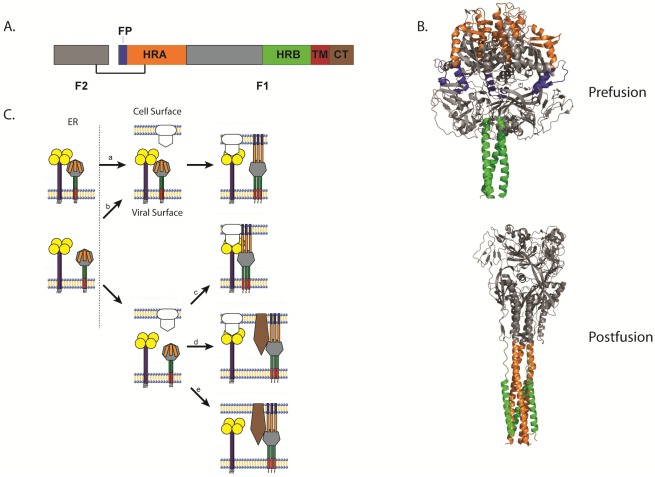
Figure 2.
(A) Schematic of the cleaved, disulfide-linked paramyxovirus fusion protein. (B) Structure of the uncleaved form of PIV5 F in its prefusion conformation [62] and structure of the hPIV3 F in its postfusion conformation [69]. (C) Schematic of the different models of paramyxovirus triggering. (a) The attachment and F proteins could interact while trafficking through the different biosynthetic compartments and dissociate upon receptor interaction with the attachment protein, allowing the F protein to trigger. (b) Alternatively, the attachment and F proteins could travel separately through the biosynthetic pathway, associate at the cell surface, and dissociate after attachment protein interaction with receptor, triggering the F protein. (c) The attachment and F proteins could also travel separately and not associate until the attachment protein interacts with its receptor. The association between the attachment protein and the F protein allows the latter to trigger. (d) Direct interaction between the attachment and F proteins may not be required for some viruses as receptor binding by the attachment protein could facilitate binding of another receptor by the F protein, allowing the F protein to trigger. (e) Finally, some paramyxoviruses do not require the expression of an attachment protein. For these viruses, the F protein binds to its receptor and then promotes membrane fusion, which in some cases can be triggered by low pH. For all images, the fusion peptide is represented in blue, heptad repeat A (HRA) in orange, heptad repeat B (HRB) in green, the transmembrane (TM) domain in red, and brown represents the cytoplasmic tail.