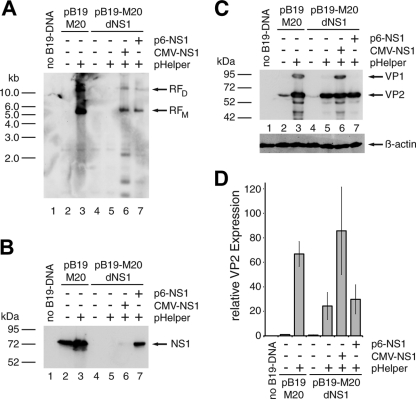
Fig 1.
Adenoviral functions induce both B19V genome replication and structural protein expression in 293 cells. 293 cells were transfected with 1 μg of SalI-linearized wild-type (pB19-M20) or NS1-negative (pB19-M20dNS1) B19V plasmid DNA. Early adenoviral functions and the B19V NS1 protein were supplied by cotransfection of 1 μg of pHelper plasmid and 0.5 μg of HCMV- or p6-driven NS1 constructs as indicated. The total amount of DNA in each transfection was adjusted to 4 μg with plasmid pCATCH containing the HCMV promoter without any downstream coding sequences. As a negative control and for monitoring the transfection efficiency, cells were transfected with 4 μg of the yellow fluorescent protein (YFP) expressing plasmid pYFP-C2 (Clontech). (A) Southern blot hybridization of DpnI-digested Hirt DNA with a biotin-11-dUTP-labeled hybridization probe from the B19V NS1 region. Arrows indicate putative monomeric (RFM) and dimeric (RFD) replicative B19V intermediates. (B and C) Western blot analysis of whole-cell extracts with the monoclonal antibodies MAB1424 recognizing the NS1 protein (B) and MAB8293 (both from Millipore) recognizing the B19V capsid proteins (C). Arrows indicate the positions of the NS1 protein and the VP1 and VP2 capsid proteins, respectively. (D) Quantitative B19V VP Western analysis with infrared fluorescence-labeled secondary antibodies as described in Materials and Methods. The VP2 expression data were normalized for the β-actin loading control and are represented as relative values, with the wild-type B19V DNA in the absence of pHelper set as 1. The bars represent arithmetic means ± the standard deviations (SD) of two independent experiments with the corresponding B19V VP ECL analysis and the β-actin control for one of these experiments shown in panel C.