Fig 1.
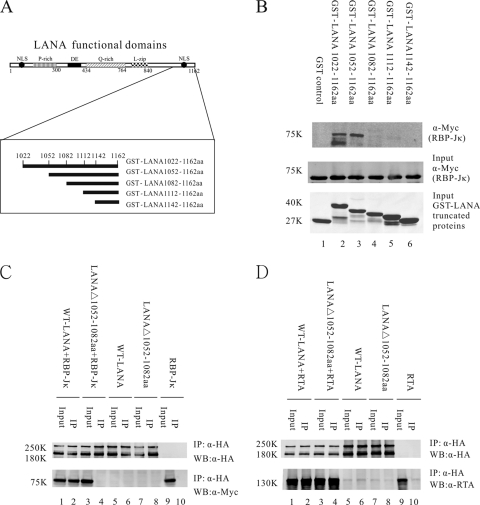
Deletion of LANA C-terminal aa 1052 to 1082 disrupts LANA interaction with RBP-Jκ but not LANA interaction with RTA. (A) Schematic diagram of LANA C-terminal mutant proteins fused with GST. Amino acids in LANA C-terminal region of aa 1022 to 1162 were deleted sequentially. (B) GST pulldown assays to detect Myc-tagged RBP-Jκ protein bound to GST-LANA mutant proteins. Equivalent amounts of GST-LANA proteins and RBP-Jκ nuclear extract were used for in vitro binding assays (middle and lower panels). Two constructs, GST-LANA(1022–1162) and GST-LANA(1052–1162), interacted with RBP-Jκ in vitro, while other constructs did not (upper panel). (C) Coimmunoprecipitation assay indicated that LANAΔ(1052–1082), with a deletion of C-terminal aa 1052 to 1082, did not interact with RBP-Jκ in 293T cells (lanes 3 and 4). Nonspecific binding was not detected (lanes 5 to 10). (D) Coimmunoprecipitation assays showed that LANAΔ(1052–1082) still interacted with RTA (lanes 3 and 4). Control panels showed no binding affinity (lanes 5 to 10). IP, immunoprecipitation; WB, Western blotting.