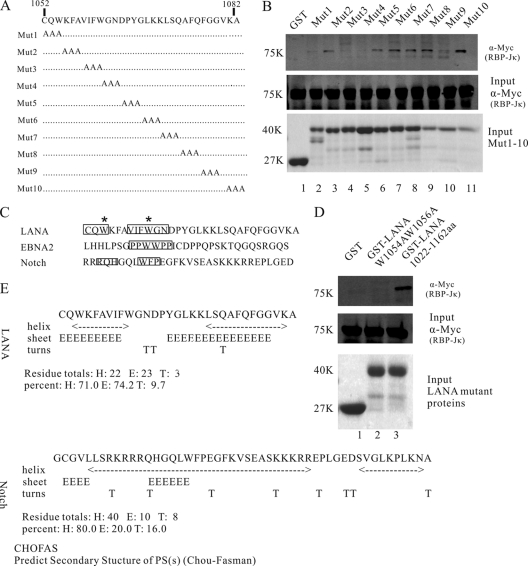
Fig 2.
Multiple amino acids in the LANA C-terminal region of aa 1052 to 1082 contribute to the LANA interaction with RBP-Jκ. (A) Schematic diagram of LANA mutations. Amino acids from LANA 1052 to 1082 were mutated to alanines (A) sequentially to determine the contribution of each site in the interaction. (B) GST-pulldown assays to detect Myc-tagged RBP-Jκ protein bound to Mut1 to Mut10. Mut1, Mut3, Mut4, and Mut9 failed to interact with RBP-Jκ, while other mutations interacted with RBP-Jκ normally. (C) Comparisons of LANA C-terminal aa 1052 to 1082 to the EBNA2 PPWWPP and Notch RAM domains. Important amino acids are boxed. Two conserved Ws are marked with asterisks. (D) GST pulldown assays to detect the interaction between GST-LANA(W1054AW1056A) and RBP-Jκ. (E) Predicted secondary structure of the LANA C-terminal aa 1052 to 1082 and the Notch RAM domain using the CHOFAS method.