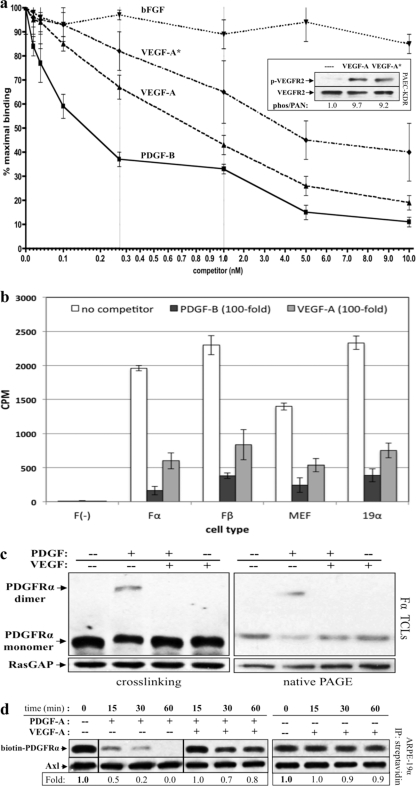
Fig 4.
VEGF-A bound to PDGFRα and prevented PDGF-dependent dimerization and internalization. (a) VEGF-A competes with PDGF-B in a dose-dependent manner for binding to PDGFRα. Fα cells were grown in 24-well plates until nearly confluent, serum starved, and subsequently incubated with 0.15 nM 125I-PDGF-B in the presence of increasing amounts of unlabeled PDGF-B, VEGF-A (freshly prepared from lyophilized powder), VEGF-A* (stored for >1 week at 4°C and/or subjected to multiple freeze-thaw cycles), or bFGF. The cells were incubated for 2 h at 4°C and then washed extensively with cold binding buffer. After incubation, the cells were harvested, and the radioactivity was quantified by using a gamma counter to determine the amount of 125I-PDGF-BB bound. The resulting IC50s were 0.16 ± 0.03 nM for PDGF-B and 0.78 ± 0.13 nM for VEGF-A. Thus, VEGF-A binds to PDGFRα with a 5.2-fold-lower affinity than its cognate ligand, although this difference remains within the same log order of binding affinity. VEGF-A* bound much more weakly (IC50 ∼4 nM) whereas bFGF did not compete. The inset shows that both VEGF-A and VEGF-A* stimulated a similar level of phosphorylation of VEGFR2 in PAE-KDR cells. (b) VEGF-A bound to PDGFRs on a variety of cell types. F, Fα, Fβ, MEFs, or ARPE-19α cells were incubated with 0.15 nM 125I-PDGF-B plus binding buffer alone (no competitor) or a 100-fold excess (15 nM) of unlabeled PDGF-B or VEGF-A. No binding was observed for F cells, which do not express PDGFRs, whereas VEGF-A bound to all other cell types: Fα and Fβ cells (expressing PDGFRα and PDGFRβ, respectively) and MEFs and ARPE-19α cells (expressing both PDGFR isoforms). (c) VEGF-A inhibited PDGF-dependent dimerization of PDGFRα. Fα cells were grown to ca. 75% confluence and serum starved overnight. The cells were then treated with DMEM alone (—), 0.5 nM PDGF-A, 0.5 nM VEGF-A, or both 0.5 nM PDGF-A and 0.5 nM VEGF-A for 10 min at 20°C. (Left panel) After treatment, the cells were placed on ice, and proximal cell surface proteins were covalently linked using a membrane-impermeable cross-linker (described in Materials and Methods). Subsequently, cells were lysed and subjected to Western analysis with anti-PDGFRα and anti-RasGAP (to assess protein loading). (Right panel) Alternatively, dimerization was assessed without cross-linking by preparing samples under nondenaturing and nonreducing conditions and performing native PAGE (described in Materials and Methods). The Western analysis was the same as for the left panel. These data indicate that PDGF-mediated dimerization of PDGFRα was inhibited by VEGF-A and that VEGF-A itself was unable to induce this response. (d) PDGF-mediated internalization of PDGFRα was inhibited by VEGF-A. ARPE-19α cells at 75% confluence were serum starved overnight. At 30 min prior to treatment, cycloheximide (2 mM) was added and retained for the duration of the experiment. The cells were then either left untreated (lane 0) or treated for the indicated times with 0.5 nM PDGF-A, 0.5 nM VEGF-A, or both 0.5 nM PDGF-A and 0.5 nM VEGF-A. After treatment, the cells were washed and placed on ice, and the cell surface proteins were biotinylated with sulfo-NHS-SS-biotin for 1 h and then quenched. Subsequently, the cells were lysed and the resulting lysates clarified. Biotinylated proteins (i.e., those remaining on the cell surface at the end of treatment) were precipitated with NeutrAvidin-agarose beads. NeutrAvidin-precipitated proteins were eluted with sample buffer and subjected to Western analysis with anti-PDGFRα and anti-Axl (the latter to assess equal loading of biotinylated proteins). The biotin-PDGFRα signal was normalized to Axl and is presented here as a ratio of the amount of receptor remaining on the cell surface over the amount of receptor remaining of the cell surface in untreated control (i.e., the absence of ligand-induced receptor internalization). The results from three independent experiments reveal that PDGF-A induced rapid internalization of PDGFRα, and this response was attenuated in the presence of an equimolar amount of VEGF-A. VEGF-A alone did not induce PDGFRα internalization.