Fig 6.
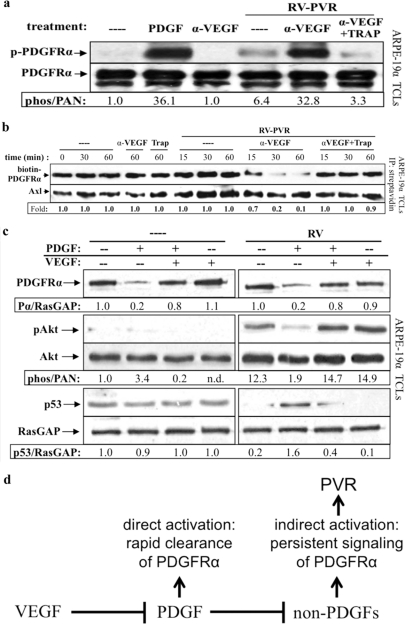
Neutralizing VEGF-A enabled vitreal PDGFs to activate PDGFRα and inhibit its indirect activation by vitreous. (a) Anti-VEGF-A promoted vitreal PDGFs to activate PDGFRα. Serum-starved ARPE-19α cells were treated 10 min at 37°C with buffer alone (–), PDGF-A (0.5 nM), or RV-PVR (0.2 ml) that was either left alone, preincubated 30 min with 25 μg of anti-VEGF-A (α-VEGF)/ml, or preincubated 30 min with 25 μg of anti-VEGF-A/ml, after which 2 μM TRAP was added. After treatment, the cells were lysed, and the resulting TCLs were subjected to the same Western analysis and quantification as described in Fig. 1. These results demonstrate that neutralizing VEGF-A significantly enhanced the ability of endogenous PDGFs in PVR vitreous to activate PDGFRα. (b) Anti-VEGF-A promoted vitreal PDGFs to internalize PDGFRα. ARPE-19α cells at 75% confluence were serum starved overnight. At 30 min prior to treatment, cycloheximide (2 mM) was added and retained for the duration of the experiment. The cells were then either left untreated (—) over the time course or treated for the indicated times in the absence of RV-PVR with 25 μg of α-VEGF-A or 2 μM TRAP/ml or treated for the indicated times in the presence of RV-PVR (0.2 ml) with nothing (—), 25 μg of α-VEGF-A/ml, or 25 μg of α-VEGF-A/ml + 2 μM TRAP. After treatment, the cells were washed and placed on ice, and cell surface proteins were biotinylated with Sulfo-NHS-SS-Biotin for 1 h and then quenched. Subsequently, the cells were lysed, and the resulting lysates were clarified. Biotinylated proteins (i.e., those remaining on the cell surface at the end of treatment) were precipitated with NeutrAvidin-agarose beads. NeutrAvidin-precipitated proteins were eluted with sample buffer and subjected to Western analysis with anti-PDGFRα and anti-Axl (the latter to assess equal loading of biotinylated proteins). The biotin-PDGFRα signal was normalized to Axl and is presented here as a ratio of the amount of receptor remaining on the cell surface over the amount of receptor remaining of the cell surface in the untreated control (i.e., absence of ligand-induced receptor internalization). The results from three independent experiments reveal that VEGF-A helps retain PDGFRα at the cell surface by antagonizing PDGF-A-mediated PDGFRα internalization. (c) Anti-VEGF-A promoted PDGF-induced direct signaling of PDGFRα and attenuated RV-induced indirect signaling responses. Serum-starved ARPE-19α cells at 75% confluence were treated with DMEM alone (—), 0.5 nM PDGF-A, 0.5 nM VEGF-A, or both in the absence (—) or presence of normal rabbit vitreous (RV) for 2 h at 37°C. After treatment, the cells were lysed and subjected to Western analysis with anti-PDGFRα, anti-phospho-Akt, anti-Akt, anti-p53, and anti-RasGAP. PDGFRα and phospho-Akt immunoblot signals were normalized to RasGAP and total Akt signals, respectively; the results are presented as the fold induction over the nonstimulated control for each pair. These results show that vitreal VEGF-A preserves RV-induced indirect signaling downstream of activated PDGFRα by antagonizing vitreal PDGFs. (d) Model of the relationship between VEGF-A, PDGFs, and non-PDGFs in PVR. While PDGFs antagonize non-PDGF-mediated activation of PDGFRα, VEGF-A promotes non-PDGF-mediated activation of PDGFRα by antagonizing PDGFs. These observations suggest that VEGF-A promotes PVR by enhancing indirect activation of PDGFRα, which results in chronic signaling that drives cellular responses intrinsic to PVR pathogenesis (45).