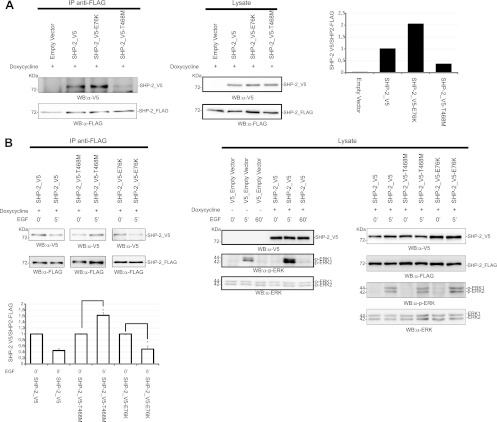
Fig 9.
Dimerization of the LEOPARD mutant SHP-2T468M shows altered kinetics upon EGF induction. 293 T-REx cells expressing the wild-type phosphatase SHP-2_FLAG were transfected with SHP-2_V5, the LEOPARD mutant SHP-2_V5-T468M, or the leukemic mutant SHP-2_V5-E76K. Expression of SHP-2_FLAG was induced for 6 h with doxycycline. Cells were starved in serum-free medium for 16 h and then lysed (A) or induced with EGF for 5 min (B). Lysates were immunoprecipitated with anti-FLAG antibody, and coimmunoprecipitated SHP-2 was revealed with anti-V5 antibody. The ratio between the coimmunoprecipitated SHP-2_V5 and the immunoprecipitated SHP-2_FLAG under starvation conditions is visualized in the upper bar chart. The lower bar chart shows the difference of dimeric content upon EGF induction, with 1 considered the level of dimers measured before EGF induction. The transfection efficiency was verified by revealing the PAGE-separated proteins with the anti-V5 antibody. Immunoprecipitation was verified with anti-FLAG antibody. The levels of SHP-2 proteins in the lysates were visualized by Western blotting either with anti-FLAG or with anti-V5. The EGF-induced phosphorylation profile of ERK1/2 proteins was monitored by probing with anti-phospho-ERK1/2 and anti-ERK1/2 antibodies. The error bars were calculated for three independent experiments (Student's t test; P < 0.05). The EGF time course (0, 5, and 60 min) shown in panel B (lysate panel) was an independent experiment to compare ERK1/2 activation in mock- and SHP-2_V5-transfected cells.