Fig 1.
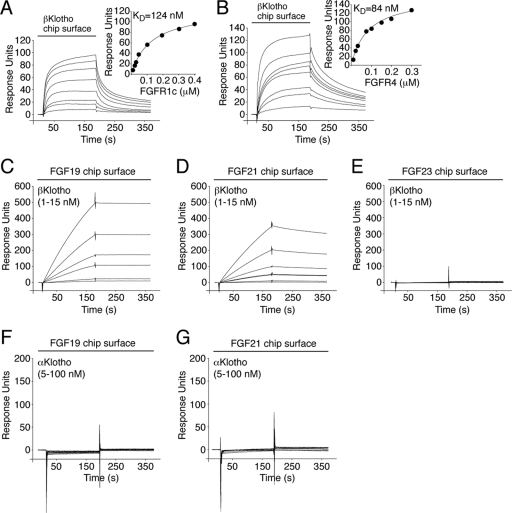
βKlotho possesses distinct high-affinity binding sites for FGF19/21 and FGFR. (A and B) Overlays of SPR sensorgrams illustrating binding of FGFR1c (A) and FGFR4 (B) to βKlotho and fitted saturation binding curves. βKlotho ectodomain was immobilized on a biosensor chip, and increasing concentrations of the ligand-binding domain of FGFR1c or FGFR4 were passed over the chip. The dissociation constants (KDs) were calculated from the saturation binding curve. (C and D) Overlays of SPR sensorgrams illustrating binding of βKlotho to FGF19 (C) and FGF21 (D). FGF19 and FGF21 were immobilized on a biosensor chip, and increasing concentrations of βKlotho ectodomain were passed over the chip. Note that for any given concentration of βKlotho, the binding response is greater on the FGF19 chip surface than on the FGF21 chip surface. Also note that the FGF19-βKlotho complex dissociates more slowly than the FGF21-βKlotho complex (compare the dissociation phases of the sensorgrams shown in panels C and D). (E) Overlay of SPR sensorgrams showing no interaction between βKlotho and FGF23. FGF23 was immobilized on a biosensor chip, and increasing concentrations of βKlotho ectodomain were passed over the chip. (F and G) Overlays of SPR sensorgrams showing no interaction between αKlotho and FGF19 (F) or FGF21 (G). FGF19 and FGF21 were immobilized on a biosensor chip, and increasing concentrations of αKlotho ectodomain were passed over the chip. The data shown in each figure panel are representative of two to three independent experiments.