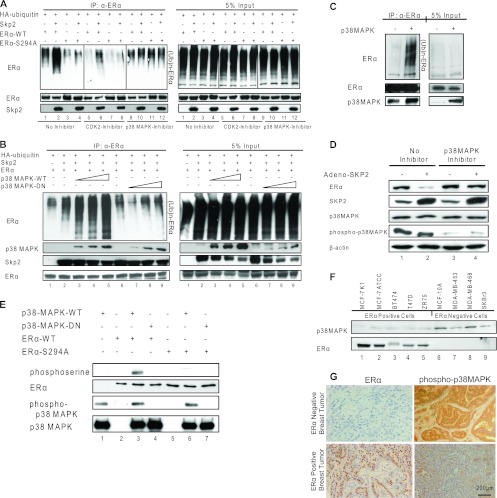
Fig 5.
p38MAPK-mediated phosphorylation of ERα at serine 294 is required for its ubiquitination by Skp2. (A) Ubiquitination assay for the ERα-WT or ERα-S294A protein in Cos-1 cells exposed to the vehicle control, the cyclin-dependent kinase 2 (CDK2) inhibitor (1 μM), or the p38MAPK inhibitor (1 μM) in the absence or presence of Skp2. (B) Ubiquitination assay for ERα in Cos-1 cells in the presence of increasing amounts of either pCMV-p38MAPK-WT or p38MAPK-DN. (C) Ubiquitination assay for endogenous ERα in MCF-7 cells infected with the control or p38MAPK adenovirus. (D) Western analysis of MCF-7 cells infected with the control or Skp2 adenovirus and treated or not with the p38MAPK inhibitor. (E) In vitro kinase assay using an active or inactive recombinant GST-p38MAPK protein with in vitro-translated ERα-WT or ERα-S294A protein as the substrate. (F) Western analysis of p38MAPK and ERα proteins in multiple breast cancer cell lines. (G) Immunohistochemical staining for phospho-p38MAPK and ERα proteins in ER-negative and -positive human breast tumors. All data are representative of 4 experiments.