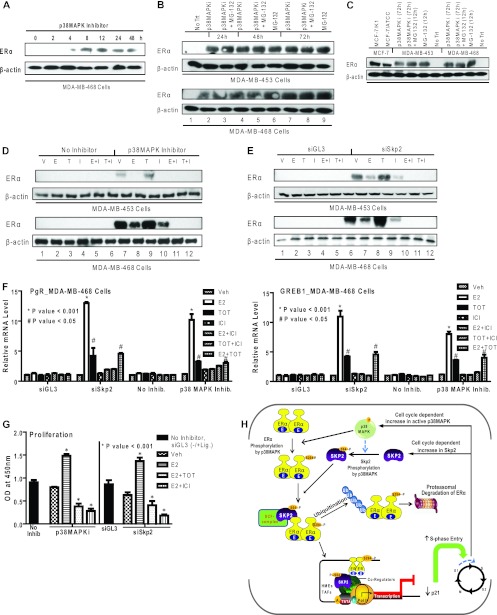
Fig 7.
Restoration of ERα protein and functional activities in ERα-negative breast cancer cells by treatment with a p38MAPK inhibitor or knockdown of Skp2. (A) Western analysis for ERα in MDA-MB-468 cells treated with a p38MAPK inhibitor (SB203580) (1 μM) for the indicated times. (B) Western analysis for ERα in MDA-MB-453 and -468 cells receiving no inhibitor treatment (vehicle only) (No Trt), the p38MAPK inhibitor alone or in combination with MG-132 (10 μM), or MG-132 (10 μM) alone. (C) Western analysis for comparison of ERα in the ERα-positive cell lines MCF-7/K1 and MCF-7/ATCC and ERα-negative cell lines MDA-MB-453 and -468 treated with the vehicle, with the p38MAPK inhibitor for 72 h with or without MG-132 for the last 12 h, or with MG-132 alone. Equal amounts of cell protein were loaded onto each lane. (D) Western analysis of MDA-MB-453 and -468 cells treated with the vehicle (V) or the p38MAPK inhibitor followed by the vehicle or ligand treatments indicated (10 nM E2 [E], 10 nM TOT [T], 1 μM ICI 182,780 [I] alone or E2 and ICI 182,780, or TOT and ICI 182,780). (E) Western analysis of ERα in MDA-MB-453 and -468 cells transfected with siGL-3 or siSkp2 followed by the vehicle or ligand treatments indicated. (F) Real-time RT-PCR analysis to monitor PgR and GREB1 mRNAs in MDA-MB-468 cells after siSkp2 or control siGL-3 treatment for 48 h or treatment with no inhibitor or the p38MAPK inhibitor for 48 h, followed by treatment with the vehicle or the indicated ligands for 24 h. (G) Proliferation of MDA-MB-468 cells in the presence or absence of the p38MAPK inhibitor or after siGL-3 or siSkp2 exposure for 48 h, followed by a 6-day treatment with the indicated ligands. Cell numbers were analyzed by a WST reagent assay. Data are from 3 experiments and are represented as means ± SD. OD, optical density. (H) Model showing the regulation of ERα turnover and functional activity by Skp2 and p38MAPK. The posttranslational modification of ERα by the p38MAPK-dependent phosphorylation of ERα at Ser-294 controls the turnover of ERα by the E3-ubiquitin ligase Skp2 and also the regulation of gene expression and enhanced cell cycle progression. Furthermore, in some ERα-negative breast cancer cells, the inhibition of p38MAPK or the depletion of Skp2 restores cellular ERα protein levels and the responsiveness to estrogen- and antiestrogen-regulated gene expression and cell proliferation.