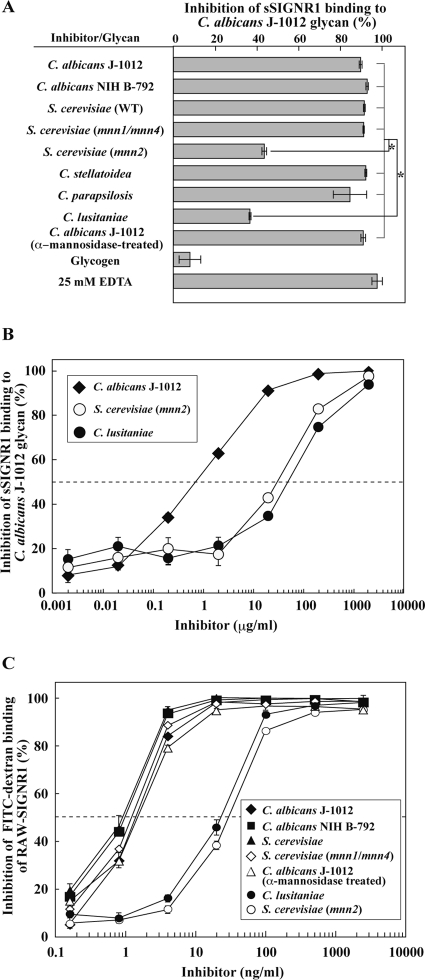
Fig 2.
Recognition of α-mannose side chains in N-glycan by sSIGNR1. (A) Inhibition analysis by lectin ELISA. Binding of sSIGNR1 to microtiter plates coated with C. albicans J-1012 glycan was analyzed in the presence of glycans (25 μg/ml) purified from various types of yeast strains. Blocking activities of inhibitors are shown as the percent inhibition of sSIGNR1 binding. (B) Titration of inhibitory activity of glycans from the indicated yeast strains for sSIGNR1 binding by lectin ELISA. Half of maximal inhibition activity was indicated by the dashed line. (C) Inhibition of FITC binding to RAW-SIGNR1 cells by glycans. Transfectants were incubated with graded doses of glycans as in panel B prior to FITC-dextran. The results are shown as percent inhibition (means ± SD of triplicate assays). *, P < 0.05 (solid lines) by Tukey's multiple-range test. The gray lines indicate no significant differences.