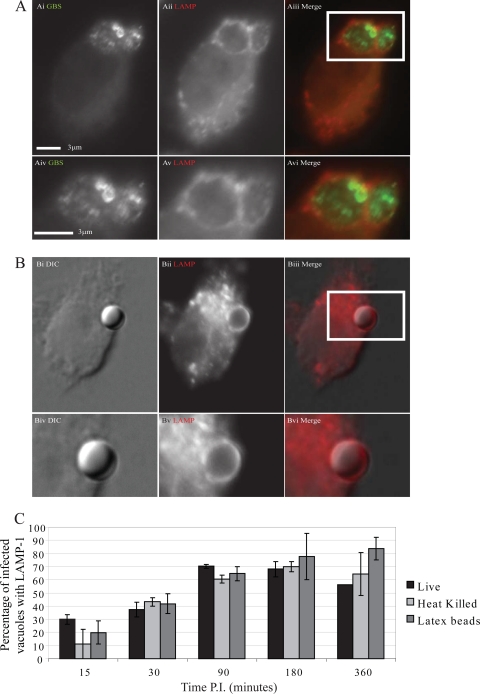
Fig 3.
Group B Streptococcus-containing phagosomes show no delay in LAMP acquisition. J774 macrophages settled on coverslips were infected synchronously with live wild-type GBS strain NEM316, heat-killed NEM316, or latex beads. Coverslips were removed at time points postinfection, fixed, and immunostained for streptococci (green) and lysosome-associated membrane protein (red). Coverslips were visualized using a Nikon Eclipse Ti microscope with a 100× DIC objective. Images shown are from 90 min postinfection. (A) Live FITC-stained GBS (i and iv), with LAMP, stained with TRITC (ii and v), acquisition. Merged images are shown in panels iii and vi. (B) Representative image of a latex bead-containing phagosome with corresponding DIC (i and iv), LAMP (iii and iv), and merged (v and vi) images. The graph (C) shows the average percentage of GBS or bead-containing vesicles which costained for LAMP. At least 50 phagosomes were scored in each independent repeat. Peak acquisition can be seen at 90 min postinfection (P.I.), with a slight decrease at 360 min postinfection in the phagosomes containing live GBS. There is no difference observed between live organisms (black bars), heat-killed organisms (light gray), and 3-μm latex beads (dark gray) (χ2 on raw data, P > 0.05), with the exception of the live organisms and latex beads at 360 min postinfection (P = 0.004). Error bars represent standard errors from three repeats.