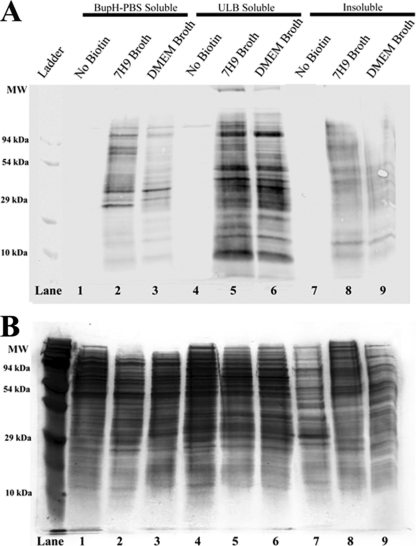
Fig 1.
Biotin labeling of M. avium subsp. hominissuis proteins separated by solubility. Protein was analyzed from three M. avium subsp. hominissuis samples, one negative control (not biotinylated) and two experimental samples (biotinylated). Protein was isolated on the basis of solubility in three consecutive extractions. The first extraction was performed in BupH-PBS and isolated primarily PBS-soluble proteins that are expected to be soluble in native conditions (lanes 1 to 3). The pellet remaining from the first extraction was resuspended in ULB, which was expected to solubilize most of the membrane and cell envelope-associated proteins (lanes 4 to 6). The pellet remaining after the extraction of ULB-soluble proteins was resuspended in Laemmli buffer and heated to 95°C for 10 min to extract as much of the remaining protein as possible (lanes 7 to 9). (A) Anti-biotin Western blotting indicates that the majority of the biotin-labeled protein was present in the ULB fraction. Some signal was detected in the insoluble fraction, indicating that some surface-exposed proteins were unidentifiable in this study. The negative control shows minimal staining, suggesting low levels of endogenous biotinylation. (B) Silver-stained SDS-PAGE gel of the same protein samples.