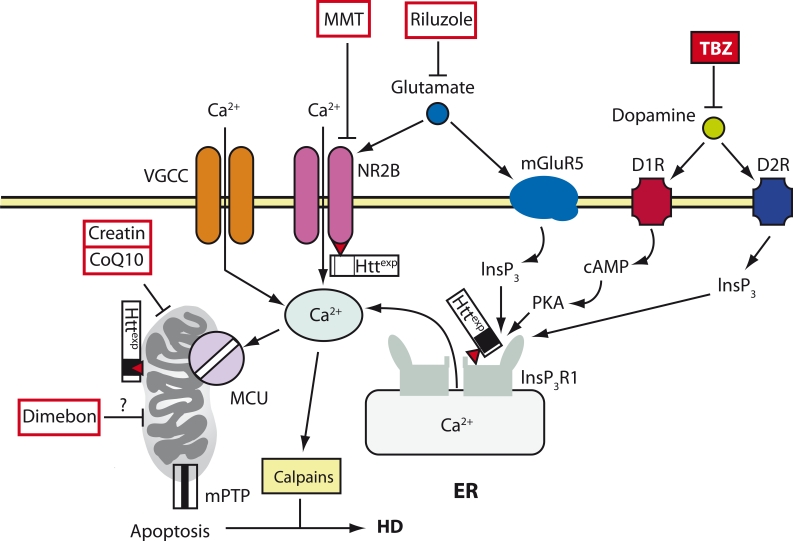
Fig. 1.
A model of Ca2+ deregulation during HD (cited from (Tang et al., 2007)). In MSNs during HD, Httexp disrupts Ca2+ signaling by three syn- ergistic mechanisms. Httexp increases the function of the NR2B-bearing NMDA receptor (probably by increasing its transport into the plasma membrane). Httexp tightly binds to the С-terminus of InsP3R1 and increases its affinity to InsP3. The low level of glutamate secreted by the neurons of the corticostriatal projection causes an excessive influx of Ca2+ via the NMDA receptor and the release of Ca2+ from the ER via InsP3R1. The additional uptake of Ca2+ into MSNs is mediated by VGCC. Dopamine excreted by the dopaminergic neurons of the mesencephalon stimulates the type-1 (D1R) and type-2 (D2R) dopamine receptors, which are highly expressed in MSN. D1R is connected with an adenylate cyclase, and together they increase the cAMP level and activate the protein kinase A (PKA). PKA enhances the glutamate-induced Ca2+ signals by increasing the activity of the NMDA receptor and InsP3R1. D2R is directly involved in the produc- tion of InsP3 and the activation of InsP3R1. The excessive uptake of Ca2+ activates calpain, which cleaves Httexp and other substrates. The excess of Ca2+ in the cytosol leads to the capture of Ca2+ by the mitochondria via MCU, which in turn induces the opening of mPTP and apoptosis. The calcium regulation of mitochondria is also disrupted due to the direct interaction between Httexp and the mitochondria. The antidopamine drug tetrabenzine (TBZ) has been approved in the United States for the symptomatic treatment of HD. The NMDA receptor antagonist memantin (MMT), the soluble “mitochondrial agent” dimebon and “mitochondrial stabilizers” creatin and coenzyme Q10 (CoQ10) are all in clinical trials. The antiglutamate agent Riluzole has passed clinical trials, but it turned out to be ineffective for HD treatment [19]