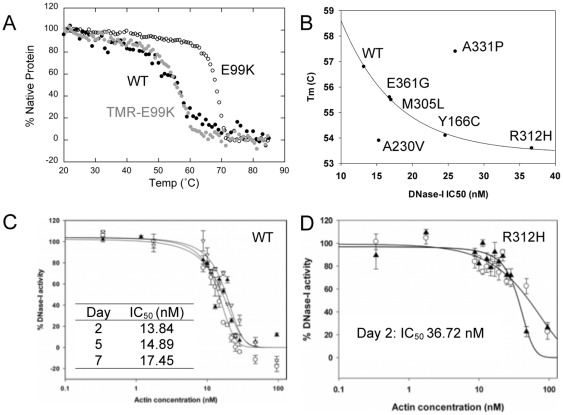
Figure 2. Intrinsic cardiac actin mutant monomer properties.
A . The melting temperature of WT ACTC (•) (black circle) was similar to that of TMR-modified E99K ACTC (•) (grey circle) (56.8±1.3°C and 56.7±0.23°C, respectively). Unmodified E99K ACTC (Ο) had a melting temperature of 68.8±0.35°C. B . Correlation of measured T m and DNase-I IC50 values. The values for five of the tested ACTC proteins fitted a single exponential decay equation (y = y0+Ae -bx, where y0 was 53.4±0.15, A was 19.27±2.65, and b was 0.131±0.01 (S.E.)) with an R2 of 0.998). Both ACTC variants with measured T m values higher than WT displayed higher DNase-I IC50 values (E99K ACTC is not shown), while the A230V ACTC displayed a lower Tm value with an IC50 similar to WT ACTC. C. DNase-I inhibition curves with WT ACTC protein show little change over time (day 2, Ο; day 5, ?; day 7, ▿). Data points are averages of triplicate measurements, with error bars showing standard deviation. D. DNase-I inhibition by R312H ACTC protein shows a higher initial IC-50 value (day 2, ?). At day 7 (Ο), the IC50 data could not be fitted because it had increased.