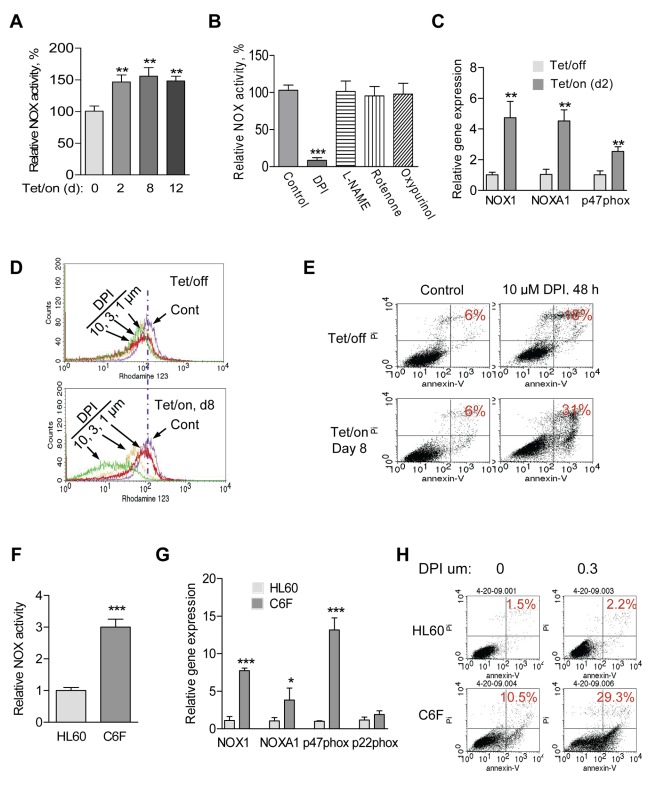
Figure 4. Cells with mitochondrial respiratory defects exhibit elevated NOX activity and are sensitive to NOX inhibition.
(A) Increase of membrane-associated NOX activity in cells with mitochondrial respiratory defects after induction of POLGdn expression (Tet/on, 2, 8, 12 d). Error bars, ±SD. ** p<0.01 (n = 3). (B) Inhibition of NOX enzyme activity by DPI. The Tet/on cells (day 8) were treated with 10 µM DPI, 100 µM L-NAME, 20 µM rotenone, or 100 µM oxypurinol for 4 h, and the membrane-associated fractions were prepared for analysis of NOX activity. Error bars, ±SD. *** p<0.001 (n = 3). (C) Increase in mRNA expression of NOX family members in Tet/on (day 2) cells, measured by qRT-PCR analysis. Error bars, ±SD. ** p<0.01 (n = 3). (D) Comparison of changes in mitochondrial transmembrane potential in Tet/off and Tet/on cells treated with DPI. Cells were pre-induced by doxycycline for 7 d and then incubated with the indicated concentrations of DPI for 24 h. Mitochondrial transmembrane potential was measured by flow cytometry using Rhodamine-123 as a potential-sensitive dye. Cells without DPI treatment were marked as control (Cont). (E) Cells with mitochondrial respiratory defect (Tet/on, day 8) were more sensitive to DPI treatment (10 µM, 48 h) compared with the Tet/off cells. Cell viability was measured by annexin-V/PI assay. (F) Increase of NOX activity in mDNA-less HL60-C6F (C6F) cells. Mean ± SD. *** p<0.001 (n = 3). (G) Increase in NOX family mRNA expression in C6F cells, measured by qRT-PCR assay. Data are shown as mean ± SD of triplicate samples from two independent experiments. * p<0.5; *** p<0.001. (H) C6F cells were more sensitive to DPI treatment. HL60 and its derived C6F cells were treated with indicated concentration of DPI for 48 h. Cell viability was measured by annexin-V/PI assay.