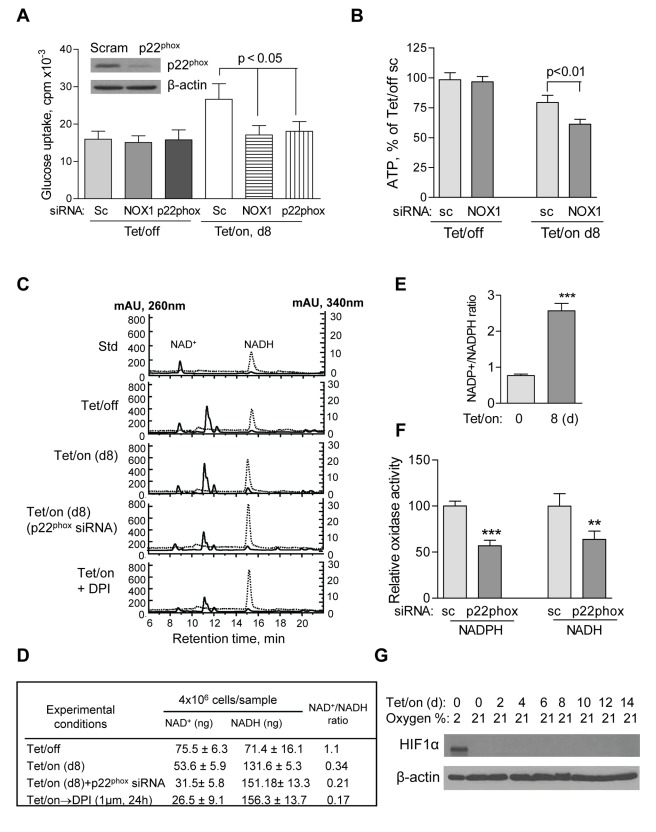
Figure 5. NOX supports glycolysis in cells with mitochondrial respiratory defects induced by POLGdn.
(A) Inhibition of glucose uptake by siRNA knockdown of NOX1 or p22phox in cells with mitochondrial respiratory defects (Tet/on, day 8), but not in cells with intact mitochondria (Tet/off). p<0.05 (n = 3). Insert: Tet/off and Tet/on (at day 4) cells were transiently transfected with p22phox siRNA and the knockdown efficiency was detected by anti-p22phox antibody using Western blot. Non-targeting control siRNA (Scram or sc) was used as negative control. (B) Effect of NOX1 knockdown on ATP contents in cells with intact mitochondria (Tet/off) and cells with mitochondrial respiratory defect (Tet/on, day 8). p<0.01 (n = 4). (C) HPLC analysis of cellular NAD+ and NADH levels. Standard NAD+ and NADH were used as references, which were monitored simultaneously at 260 nm and 340 nm, respectively. A lower NAD+ content was detected in the Tet/on cells with mitochondrial respiratory dysfunction and higher glycolytic activity that consumed more of NAD+. Inhibition of NOX activity by p22phox siRNA and DPI resulted in further decrease in cellular NAD+ level. (D) Quantitation of intracellular NAD+ and NADH in triplicate experiments, using HPLC method as described above. (E) Effect of POLGdn expression on cellular NADP+/NADPH ratio. *** p<0.001. (F) Knockdown of p22phox decreased NADPH/NADH oxidase activity. Comparing to the control siRNA knockdown in Tet/on (d8) cells, p22phox knockdown significantly decreased cellular NADPH/NADH oxidase activity using either NADHP or NADH as substrate. All error bars, ±SD. ** p<0.01; *** p<0.001 and n = 3. (G) HIF-1α is not stabilized following POLGdn induction. Protein level of HIF-1α in cells at the indicated time points after POLGdn induction was assayed by Western blot analysis. Tet/off cells with 2% oxygen were used as positive control. β-actin was used as a loading control.