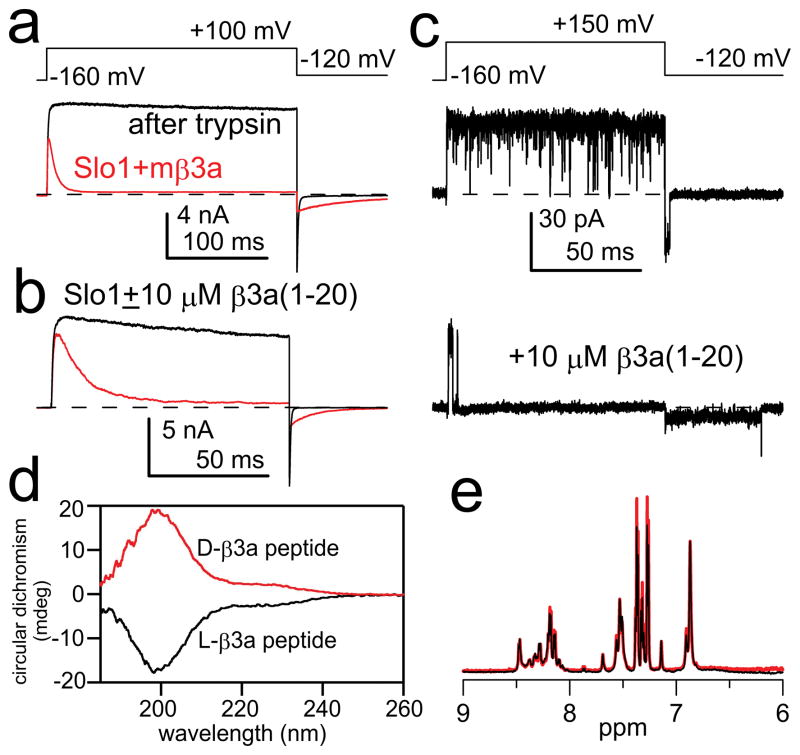
Figure 1. Intrinsically disordered β3a-N-terminal peptide mimics the two-step inactivation produced by the tethered β3a N-terminus.
(a) Intact β3a subunits mediate trypsin-sensitive inactivation and tail current prolongation of BK channels. Red trace: BK α+β3a currents with the indicated protocol and 10 μM Ca2+; black trace: after trypsin. (b) β3a(1-20) peptide (red) produces inactivation and slows BK tail currents. (c) Single BK channel in the absence (upper) and presence (lower) of β3a(1-20) peptide. Peptide produces block and a noisy long duration low amplitude openings prior to unblock. (d) Circular dichroism spectra of L- (black) and D- β3a-peptides (red) are identical except for expected opposite sign. Minimum below 200 nm is characteristic of random coil conformation. (e) Solution NMR of β3a 21-mer peptides (black: L-peptide; red: D-peptide) shows minimal chemical shift dispersion in the amide region.