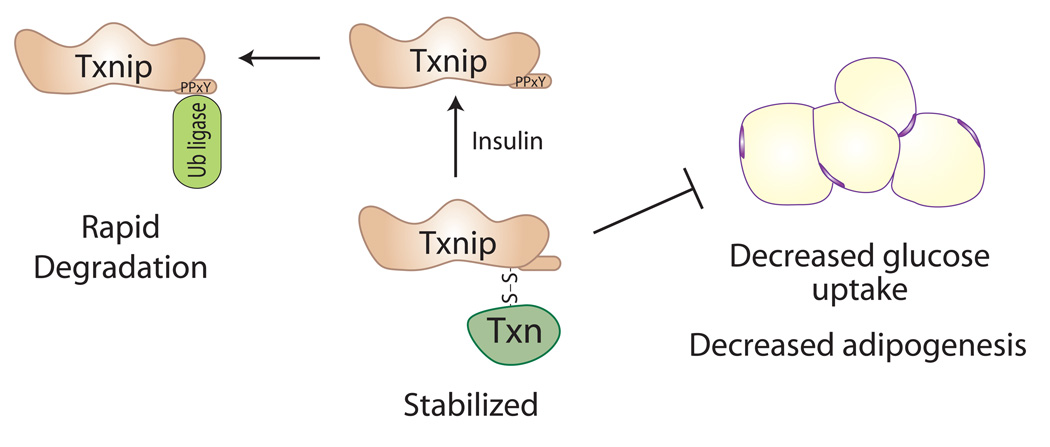
Figure 2. Regulation of Txnip by Thioredoxin.
Txnip was originally identified as a regulator of redox stress that binds thioredoxin and thereby inhibits its oxidoreductase activity. Txnip also has critical roles in blocking cellular glucose uptake and glycolytic metabolism, and in inhibiting adipogenesis. This led to the hypothesis that Txnip regulates metabolism by inhibiting thioredoxin and increasing oxidative stress. However, based on recent results, we now propose the reverse: that thioredoxin enhances Txnip’s function by increasing its stability [27, 35]. In this model, Txnip is subject to rapid protein degradation through recruitment of E3 ubiquitin ligases to its C-terminal PPxY motifs (for example, by treatment with insulin). In contrast, binding of Txnip to thioredoxin, perhaps by reducing accessibility of the PPxY motifs, leads to a Txnip-thioredoxin complex that resists degradation and thereby enhances Txnip functions.