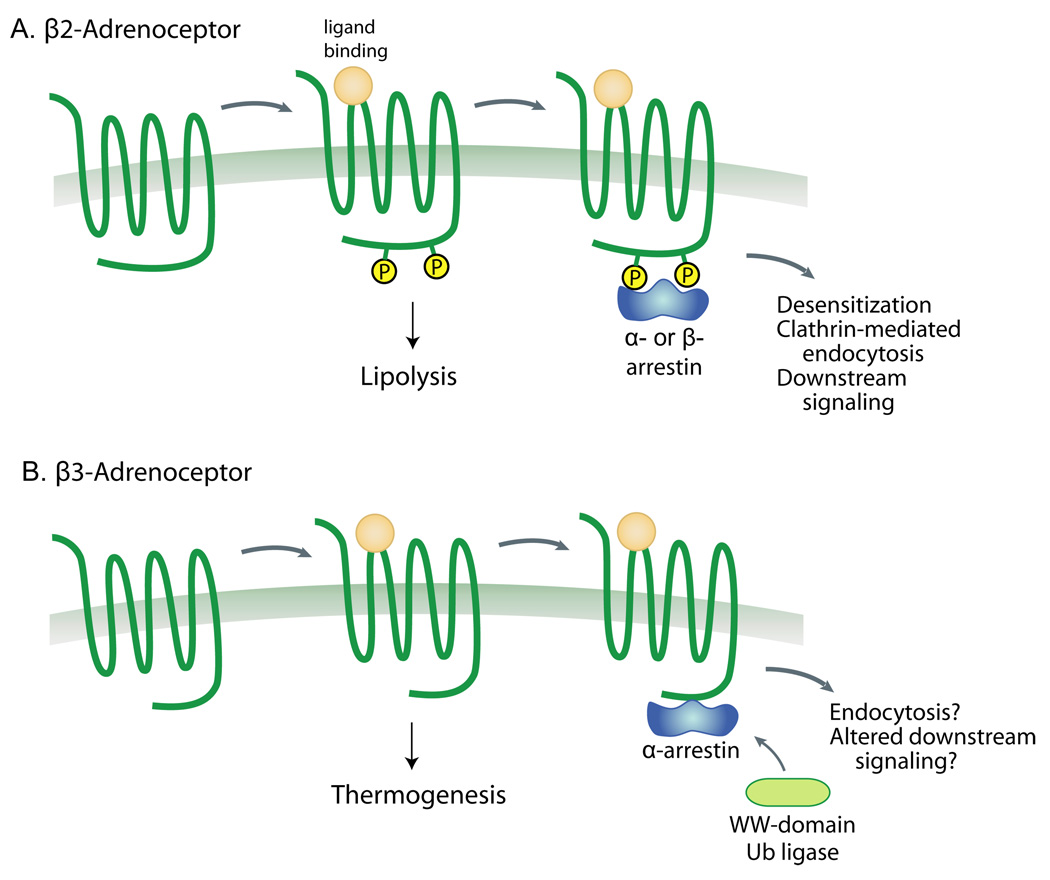
Figure 3. Schematic representation of open hypotheses for alpha and beta arrestin actions on beta-adrenergic receptors in adipose tissue.
A) Activation of the adipose tissue β1 and β2 adrenergic receptors causes a Gs-mediated increase in cAMP levels, stimulating lipolysis. This receptor has a classical paradigm for desensitization in which beta-arrestins are recruited to the active phosphorylated receptor, leading to ubiquitination and clathrin-mediated endocytosis [11]. We hypothesize that alpha-arrestins such as Arrdc3 may serve similar roles in this process. B) In contrast, the β3-adrenergic receptor is not phosphorylated and is largely not downregulated, at least in vitro. Furthermore, signaling is through Gi activation of ERK and through G-protein-independent recruitment of Src [63]. Whereas beta-arrestins are not recruited to the β3-adrenergic receptor, we hypothesize that alpha-arrestins may be able to either promote receptor ubiquitination or alter downstream signaling, thereby regulating thermogenesis in adipose tissue.