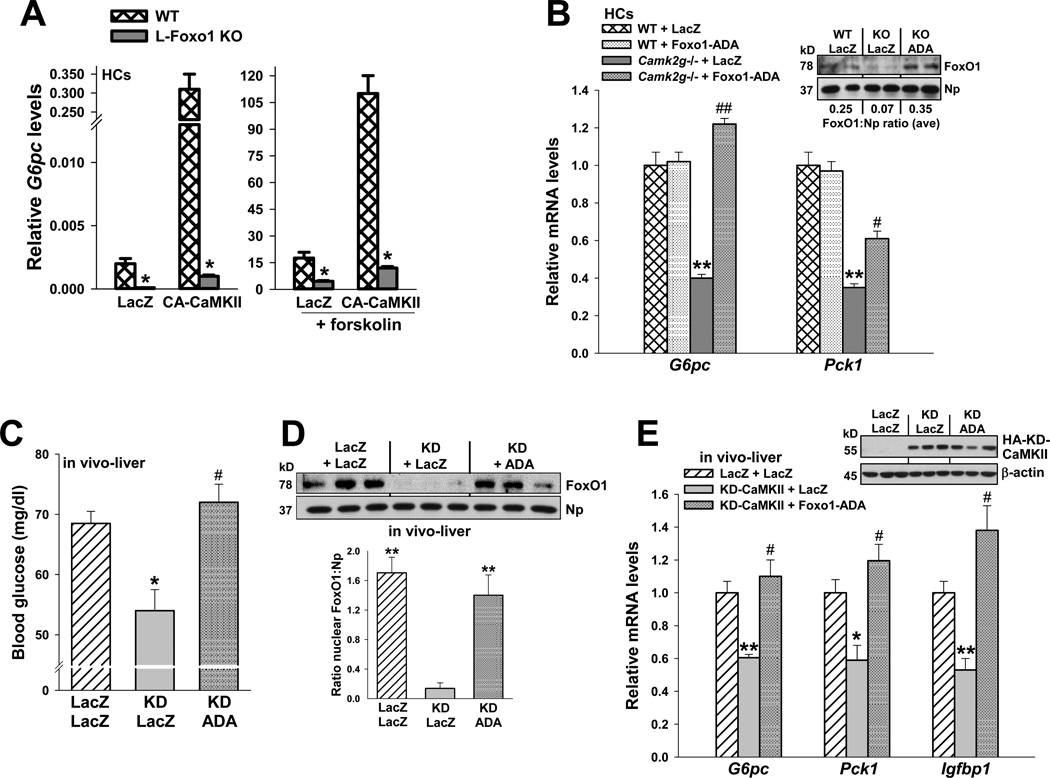
Figure 5. Impairment of glucose metabolism by CaMKII inhibition is rescued by transduction with constitutively nuclear FoxO1-ADA.
(A) HCs from wild-type or L-FoxO1 knockout mice were transduced with adeno-LacZ or CA-CaMKII. The cells were serum-depleted overnight and then incubated for 5 h in the absence or presence of forskolin (10 µm) in serum-free media. RNA was assayed for G6pc mRNA (*P < 0.001; mean ± S.E.M.). (B) HCs from WT and Camk2g−/− mice were administered adeno-LacZ or FoxO1-ADA at an MOI of 0.2. Cells were serum-depleted overnight and then incubated for 5 h with 10 µM forskolin in serum-free media. RNA was assayed for G6pc and Pck1 mRNA (**P < 0.01 vs. WT groups; #P < 0.05 and ##P < 0.01 vs. Camk2g−/−/LacZ group; mean ± S.E.M.). Inset, the nuclei from a parallel set of cells were probed for FoxO1 and nucleophosmin by immunoblot; the average densitometric ratio appears below each pair of lanes. (C–E) 8-wk/o WT mice were administered adeno-LacZ or KD-CaMKII, and then one day later, half of the adeno-KD-CaMKII mice received adeno-FoxO1-ADA, while the other half received adeno-LacZ control. Blood glucose levels were assayed at day 5 after a 12-h fast (*P < 0.05 vs. LacZ/LacZ; #P < 0.05 vs. KD/LacZ; n = 5/group; mean ± S.E.M.), and liver was assayed for nuclear FoxO1 protein (**P < 0.01 vs. KD/LacZ; mean ± S.E.M.); G6pc, Pck1 and Igfbp1 mRNA (*P < 0.05 and **P < 0.01 vs. LacZ/LacZ; #P < 0.05 vs. KD/LacZ; n = 3/group; mean ± S.E.M.). The inset to panel E shows the level of hemagglutinin (HA)-tagged KD-CaMKII protein (anti-HA immunoblot).